Formula sheet guide: FormulaCheatSheet

MAKING SENSE OF THE EQUATION SHEET
Interference & Diffraction
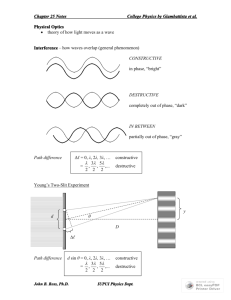
INTERFERENCE
d sin . Equation for path length difference. r1 – r2 is completely general. Use sin only when the two sources are far away from the observation point.
is completely general whenever you have waves from two sources interfering.
2
d sin
d
d y
applies to interference from multiple slits. is the phase difference between
2 L waves from successive slits at the point of observation. d is the slit separation. is the wavelength. is the position on the screen measured as an angle. y is the position on the screen measured as a distance. L is the distance from the slits to the screen. sin that: sin n d
I 4 cos 2
applies to interference from multiple slits.
successive peaks.
n d
for small angles and that
is the angular position of the n
d
where
applies only to the superposition of 2 waves. th order peak. Note
is the angular separation between
DIFFRACTION a
a sin applies to diffraction. a
is the path length difference between the top and bottom of the slit of width a .
2
...
applies to diffraction. Here bottom of the slit.
is the phase difference between the waves coming from the top and the sin m a
applies to diffraction. is the angular position of the m th order minimum caused by diffraction.
INTERFERENCE PLUS DIFFRACTION
I
1
I
sin
/ 2
/ 2
2
gives the shape of the diffraction pattern (the envelope).
I
N
I
sin
N / 2 sin
/ 2
2
gives the shape of the interference pattern (the peaks). N is the number of slits.
Note that: I I
sin
/ 2
/ 2
sin
N / 2 sin / 2
2
gives the total intensity pattern.
RESOLUTION OF LENSES, GRATINGS, ETC
a
is the minimum angular separation of two objects resolvable through a 1D slit of width a.
0
1.22
D is the minimum angular separation of two objects resolvable through a lens or circular aperture of diameter D. c
can also be taken to mean the minimum resolvable angle.
min
1
applies to resolution of two interference peaks through a diffraction grating.
Nm resolvable wavelength difference. N is the number of slits. m is the order of the peak.
is the minimum
MAKING SENSE OF THE EQUATION SHEET
Quantum Physics, Part I
ENERGY & MOMENTUM
KE max
eV stop
hf off the metal is KE max
. V stop the metal.
f
0
applies to the photoelectric effect. The maximum kinetic electrons coming
is the stopping voltage. hf is the energy of the photon. is the work function of
Note: Multiplying any voltage V by electric charge e gives energy in eV numerically equal to the voltage.
For example: If V 69 volts, then
69 volts
69 eV .
KE
1
2 mv 2 p 2
2 m
gives the kinetic energy for any massive particle. Note that a photon is not a massive particle.
E pc hf hc
wavelength.
gives the energy of a photon. For
applies to both massive particles and photons.
use nanometers for
KE p 2
h
2 m 2 m
2
2
gives the kinetic energy of any massive particle.
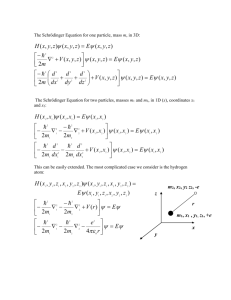
SCHRODINGERS EQUATION
i
t
KE
2
2
is for electrons.
is the time dependent schrodinger equation. Here capital psi is a function of
2 x and t.
2
is the time dependent solution to the schrodinger equation. Lowercase psi
is solution to the time independent schrodinger equation.
2
2
2
2
E is the time independent schrodinger equation. E is the energy of the particle.
*
2
is the probability density function, it gives the probability per unit length that the particle can be found at x. The * denotes complex conjugate.
P ab
a b 2 dx
Ae
gives the probability that the particle can be found between x=a and x=b.
t
is the solution to the schrodinger equation for a free particle (the potential energy U(x) is zero).
Note that E , the energy of the particle.
2 m
is the Heisenberg uncertainty principle. The uncertainty in momentum multiplied by the uncertainty in position must be greater than or equal to .
n
2
L sin n
L x gives the n th state wavefunction for a particle in an infinite square well of length L.
E n
2
2
n
2
h 2
8 mL 2
n 2 E n 2 gives the n th state energy for a particle in an infinite square well of length L. n 2 L gives the n th state wavelength of the wavefunction for a particle in an infinite square well of length L.
MAKING SENSE OF THE EQUATION SHEET
Quantum Physics, Part II
THE FINAL STUFF
T e 2 KL where K 2
2 m
U o
E
2
T is the probability that a particle of energy E can tunnel through a potential energy barrier of length L and height
U
0
. t
0
2 h
E
2
E
1
This equation gives the half-period of the time-dependent wavefunction that results from a superposition of two stationary states.
e 2 r
The “coulomb potential”, or in other words, the potential that an electron in a hydrogen atom “feels”. e is the electric charge (and here we assume there is a single proton; otherwise it would be e(Ze)). r is the distance to the nucleus. 1/ 4
0 is a constant.
8 abc sin
n
1 a
x
n
2 b
y
n
3 c
z
This is the wavefunction for a particle in an 3-dimensional infinite square well of lengths a, b, c, in the x, y, z directions respectively. n1, n2, and n3 are independent of each other, but must be >1.
3
8 h 2
n 2
1 n 2
2 m a 2 b 2 c n 2
3
2
Allowed energies for the particle in 3D infinite square well.
1 s
r
1 e
0
a
0
3
The ground state wavefunction for the electron in the hydrogen atom. a
0 is the bohr radius.
E n
13.6 eV n 2
: Energy levels for the hydrogen atom.
E n
13.6 eV
Z n 2
2
Energy levels for an electron subject to Z positive charges. Note that Z=1 gives the hydrogen equation.
4 r
2 dr
You probably won’t have to use this equation. What it means it that probability per unit of radial distance is equal to
nlm
r
4 r
R nl
2
. To find probability over a whole range of r, integrate with respect to r.
lm
General form of hydrogen wavefunctions. R is the radial wavefunction and Y is the spherical harmonic. They are independent of each other.
L 2 1
2
Very important. L is total angular momentum. l is the familiar quantum number.
L z
m
Also important. Angular momentum in z direction is proportional to m quantum number.
, , Y
10
= …
The spherical harmonics for l =0 and l =1.
U B
Potential energy of a particle in a magnetic field is equal to magnetic moment times field strength.
F z
z dB z dz
: Follows directly from above. Take derivate w.r.t z. e
z
S z Magnetic moment of electron. e is electric charge. m e
is mass. S z
is the spin of the electron m e
E n in the z direction.
n
1
2
: energy levels of the harmonic oscillator.
S 2 1
2
: S is the spin angular momentum. s is the spin quantum number.
S z
m s
: S z
is the z component of the spin angular momentum. m s
is another quantum number related to spin s , just like m l
relates to l .