Document 10291417
advertisement

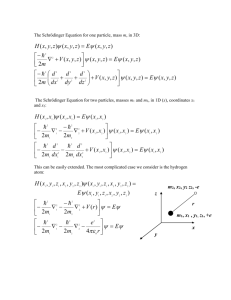
5.61 Fall 2004 Lecture #7 page 1 SOLUTIONS TO THE SCHRÖDINGER EQUATION Free particle and the particle in a box Schrödinger equation is a 2nd-order diff. eq. () h2 x + V x x = E x 2m x 2 2 () () () () () We can find two independent solutions 1 x and 2 x The general solution is a linear combination () () () x = A1 x + B 2 x () () A and B are then determined by boundary conditions on x and x . () Additionally, for physically reasonable solutions we require that x and () x (I) be continuous function. V( x ) = 0 Free particle () h2 x = E x 2m x 2 2 Define 2mE k = 2 h 2 h2 k 2 or E = 2m p2 V x = 0, E = 2m () de Broglie p= h () k= 2 p 2 = h2 k 2 p = hk 5.61 Fall 2004 Lecture #7 x 2 2 () (x) ( ) ( ) x = Acos kx + B sin kx with solutions ( ) = k 2 x The wave eq. becomes Free particle page 2 no boundary conditions h2 k 2 any A and B values are possible, any E = possible 2m So any wavelike solution (traveling wave or standing wave) with any wavelength, wavevector, momentum, and energy is possible. (II) Particle in a box () V (x) = 0 ( x < 0, x > a ) (0 x a ) V x = V( x ) 0 0 a x () Particle can’t be anywhere with V x = ( ) x < 0, x > a = 0 For 0 x a , Schrödinger equation is like that for free particle. () h2 x = E x 2m x 2 2 ( ) = k 2 x x 2 2 (x) () with same definition k2 = 2mE h2 or E = h2 k 2 2m 5.61 Fall 2004 Lecture #7 () page 3 ( ) ( ) x = Acos kx + B sin kx again with solutions But this time there are boundary conditions! () Continuity of x (i) () () () 0 = a = 0 () () 0 = Acos 0 + B sin 0 = 0 () A=0 ( ) a = B sin ka = 0 (ii) Can’t take B = 0 (no particle anywhere!) Must have ( ) sin ka = 0 ka = n n = 1, 2, 3,... k is not continuous but takes on discrete values k = n a Thus integer evolves naturally !! So solutions to the Schrödinger equation are n x 0 x a = B sin a ( ) n = 1, 2,3,... These solutions describe different stable (time-independent or “stationary”) states with energies h2 k 2 E= 2m n2 h2 En = 8ma 2 Energy is quantized!! And the states are labeled by a quantum number n which is an integer. Properties of the stationary states 5.61 Fall 2004 (a) Lecture #7 page 4 The energy spacing between successive states gets progressively larger as n increases 25EE= 51 2 2 2% h " En+1 ! En = n + 1 ! n #$ &' 8ma2 ( 16EE= 41 ) ( ) En+1 ! En = 2n + 1 E1 9EE= 31 4EE= 2218Ehma= 21 (b) () The wavefunction ! x is sinusoidal, with the number of nodes increased by one for each successive state ) (3 nodes) ( ) ( 2 nodes) ! 4 = B sin 4" x a !3 = 2a 3 ! 3 = B sin 3" x a !n = 2a n # nodes = n - 1 a!= 2 !1 = 2a (c) ( !4 = a 2 ( ) (1 node ) = B sin (" x a ) (0 nodes) ! 2 = B sin 2" x a !1 0 a x The energy spacings increase as the box size decreases. E! 1 a2 5.61 Fall 2004 Lecture #7 page 5 We’ve solved some simple quantum mechanics problems! The P-I-B model is a good approximation for some important cases, e.g. pi-bonding electrons on aromatics. Electronic transitions shift to lower energies as molecular size increases !