What is a Nanometer?
advertisement
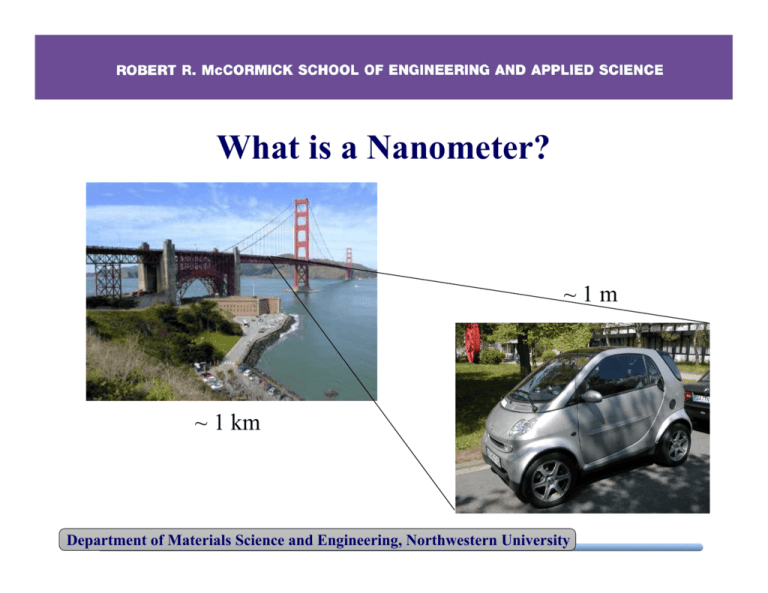
What is a Nanometer? ~1m ~ 1 km Department of Materials Science and Engineering, Northwestern University What is a Nanometer? ~ 10 mm ~1m Department of Materials Science and Engineering, Northwestern University ~ 1 mm What is a Nanometer? ~ 10 µm ~ 10 mm Department of Materials Science and Engineering, Northwestern University What is a Nanometer? ~ 1 µm ~ 10-20 µm ~10 mm ~ 1 mm Department of Materials Science and Engineering, Northwestern University What is a Nanometer? ~ 1 nm ~ 1 µm Department of Materials Science and Engineering, Northwestern University Viruses and Bacteria Cyanobacterium thin section (blue-green algae) Human papillomavirus ~50 nm ~5 µm Department of Materials Science and Engineering, Northwestern University What is a Nanometer? Department of Materials Science and Engineering, Northwestern University Size & Scale Reference Points nano 10-9 micro 10-6 milli 10-3 (meter) 100 Department of Materials Science and Engineering, Northwestern University kilo 103 What is nanotechnology? Definitions of nanotechnology on the Web: • Technology development at the atomic, molecular, or macromolecular range of approximately 1-100 nanometers to create and use structures, devices, and systems that have novel properties. plan2005.cancer.gov/glossary.html • Anything that is made up of components that are fabricated at the scale of 100 nanometers or less. nue.clt.binghamton.edu/intro1_6.html •the branch of engineering that deals with things smaller than 100 nanometers (especially with the manipulation of individual molecules) wordnet.princeton.edu/perl/webwn Department of Materials Science and Engineering, Northwestern University It matters how you slice it! Slice into smaller cubes 1cm 1cm 1cm Area = 6 x (1 cm)2 x 1 cube 1mm = 6 cm2 Area = 6 x (0.1 cm)2 x 1000 cubes = 60 cm2 Department of Materials Science and Engineering, Northwestern University 1mm 1mm It matters how you slice it! 7000 2 total surface area (m ) 6000 5000 4000 3000 2000 1000 0 1.E-09 1nm 1.E-08 10 nm 1.E-07nm 100 1.E-06 1 µm 1.E-05 10 µm cube edge 1.E-04 100 µm 11.E-03 mm Department of Materials Science and Engineering, Northwestern University 11.E-02 cm Lots of surface atoms! 0.5 0.45 Assuming surface atoms within 0.1 nm of surface fraction of surface atoms 0.4 0.35 0.3 0.25 Onset of “nanoscale” 0.2 0.15 0.1 0.05 0 1.E-09 1nm 1.E-08 10 nm 1.E-07 100 nm 1.E-06 1 µm 1.E-05 10 µm cube edge 1.E-04 100 µm 11.E-03 mm Department of Materials Science and Engineering, Northwestern University 11.E-02 cm Materials Science & Engineering ce n e i c S s l a i r Mate Processing NanoStructure Ma Properties ng E s l teria g n i r e ine Department of Materials Science and Engineering, Northwestern University Size-Dependent Properties At the nanometer scale, properties become size-dependent. For example, (1) (2) (3) (4) (5) (6) Chemical properties – reactivity, catalysis Thermal properties – melting temperature Mechanical properties – adhesion, capillary forces Optical properties – absorption and scattering of light Electrical properties – tunneling current Magnetic properties – superparamagnetic effect Æ New properties enable new applications Department of Materials Science and Engineering, Northwestern University Surface Energy Surface atoms possess more energy than bulk atoms. Consequently, surface atoms are more chemically reactive. Nanoparticles possess enhanced chemical reactivity. Example: NASA is exploring aluminum nanoparticles for rocket propulsion due to their explosiveness Department of Materials Science and Engineering, Northwestern University Nanoparticle Catalysts Macroscopic gold is chemically inert. Gold nanoparticles are used to catalyze chemical reactions. Example: Reduced pollution in oxidation reactions (i.e., environmentally friendly) Nanoparticle Catalysis Resarch Group, Tsukuba, Japan http://unit.aist.go.jp/isc/english_ver/each_groups_e/nano-cat_e/nano-cat_e.htm Department of Materials Science and Engineering, Northwestern University Alka Seltzer Missiles Water Lid Department of Materials Science and Engineering, Northwestern University Mentos and Diet Coke • • • • www.youtube.com/watch?v=5Zh1jYN2JPs Part chemical (surfactant effect) Part physical (nano-pores on the surface) Lots of gas!!! Department of Materials Science and Engineering, Northwestern University Size-Dependent Properties At the nanometer scale, properties become size-dependent. For example, (1) (2) (3) (4) (5) (6) Chemical properties – reactivity, catalysis Thermal properties – melting temperature Mechanical properties – adhesion, capillary forces Optical properties – absorption and scattering of light Electrical properties – tunneling current Magnetic properties – superparamagnetic effect Æ New properties enable new applications Department of Materials Science and Engineering, Northwestern University Macroscale Melting Temperature At macroscopic length scales, the melting temperature of materials is size-independent. For example, an ice cube and a glacier both melt at the same temperature (32ºF). Department of Materials Science and Engineering, Northwestern University Nanoscale Melting Temperature Nanocrystal size decreases surface energy increases melting point decreases e.g., 3 nm CdSe nanocrystal melts at 700 K compared to bulk CdSe at 1678 K Department of Materials Science and Engineering, Northwestern University Predicted melting temperature reduction: 0 -50 ∆Tm -100 Gold, Tm=1336K γs-l=0.132 J/m2 -150 -200 -250 -300 1.E-09 1nm 101.E-08 nm 1.E-07 100 nm 1.E-06 1 µm 1.E-05 10 µm 1.E-04 100 µm Particel Size Particle Department of Materials Science and Engineering, Northwestern University 11.E-03 mm Summary • Because of the increasing surface-to-volume ratio, the fraction of atoms at particle surfaces dramatically increases at the nanoscale • Surface-dependent properties are therefore most evident at the nanoscale, e.g., catalysis of chemical reactions • One example of surface-dependent thermodynamic property changes at the nanoscale is the dramatic decrease in the melting temperature of nano- vs. macro-sized particles • This Nano-Thermo Unit is dedicated to quantifying those changes Department of Materials Science and Engineering, Northwestern University








