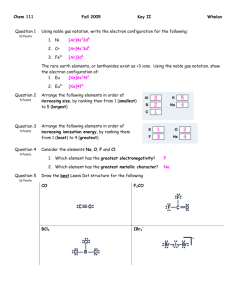
ond 1. Which of these molecules is not polar? A) H20
advertisement

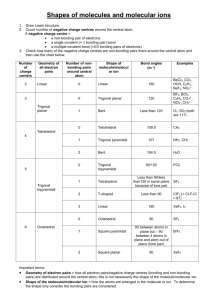
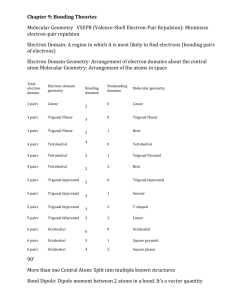
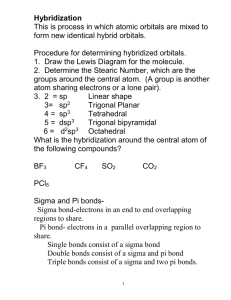
MOLECULAR GEOMETRY AND BONDING THEORY 165 Multiple Choice Questions 1. Which of these molecules is not polar? A) H20 ond C02 C) N02 ule ill <"•*• >^j fei-y'•"?•• ;™&x.'., D) $&2 E) NH3 2. Which species contains a central atom with sp2 hybridization? A) C2H2 D) BrL 3. For CIF3, the electron domain geometry ofCl and the molecular geometry are, respectively, A) trigonal planar and trigonal planar. B) trigonal planar and trigonal bipyramidal. C) trigonal bipyramidal and trigonal planar, trigonal bipyramidal and T-shapcd. E) trigonal planar and T-shaped. 4. The size of the H-N-H bond angles of the following species increases in which order? A) NH3 <NH;<NH2 B) NH3 <NH2- <NH; NH-<NH} D) <NH; NH;<NH;<NH} E) NH;<NH, • 5. What is the molecular geometry and polarity of the BF$ molecule? A) trigonal pyramidal and polar Don 166 TOPIC B) trigonal pyramidal and nonpolar C) trigonal planar and polar trigonal planar and nonpolar E) T-shaped and polar 6. In which species is the F-X-F bond angle the smallest? A) NF, B) C) BrF3 \- E) OF, 7. Which set does not contain a linear species? A) C02/SO,,N02 B) H2OfHCN, Bel, C) OCN-, C,H2, OF, D) I;, BrFy SCNH2S, C102, NH8. The hybrid orbitals of nitrogen in Nf>4 are A) sp 1~ C) sf D) dsp3 E) How many sigma and how many pi bonds are in CH2=CHCH2CCHj|j 5 sigma and 2 pi. 8 sigma and 4 pi. 11 sigma and 2 pi. 11 sigma and 4 pi. 13 sigma and 2 pi. I ^^ 7j£^fcaid!R~r-£ '* • J K^*;'^™'^- -,- . • *'•* *;«r?«£'>" '"•*jD^-vsi -sfe^s.fMl^-pa. MOLECULAR GEOMETRY AND BONDING THEORY 20. What is the best estimate of the H-O-H bond angle in H3O? A> ,«W H 1" | ; „. 207° Q 204.5" V D) 226° 5 E) 120' ' Free Response Questions s 2. Consider the chemical species IF5and IF*. a. Draw the Lewis structure and make a rough three-dimensional sketch of each species. b. Identify the orbital hybridization, the electron domain geometry and the molecular geometry of each. c. Identify the approximate bond angles of each species. d. Predict which, if any is a polar species. Justify your answer. e. Predict the most probable oxidation number of the iodine atom in each species. Give an example of another chemical species having the same oxidation number as IF*. f. Would you expect that the conversion ofIF5 to IF* to be exothermic or endothermic? Explain. 2. Consider each of these molecules: a. Draw the Lewis structure for each molecule and identify the orbital hybridization of each carbon atom.b. Specify the geometry of each central carbon atom. c. Write a balanced chemical for the complete combustion of each molecule. d. Use the following bond enthalpies to determine the heat of combustion of each molecule. Specify your answer in kj/mol. Bond kl/mol bond U/mol C-H 423 O-H 463 C-C 34S 0, 495 C=C 624 C=0 799 x-» S39 c-o 353 /-~i L=L 167
![Which is the correct Lewis structure for the nitrate ion, [NO3]– ? a) b](http://s3.studylib.net/store/data/008121614_1-3f41411d21eef682c95d3c7778684719-300x300.png)