'~'1
advertisement
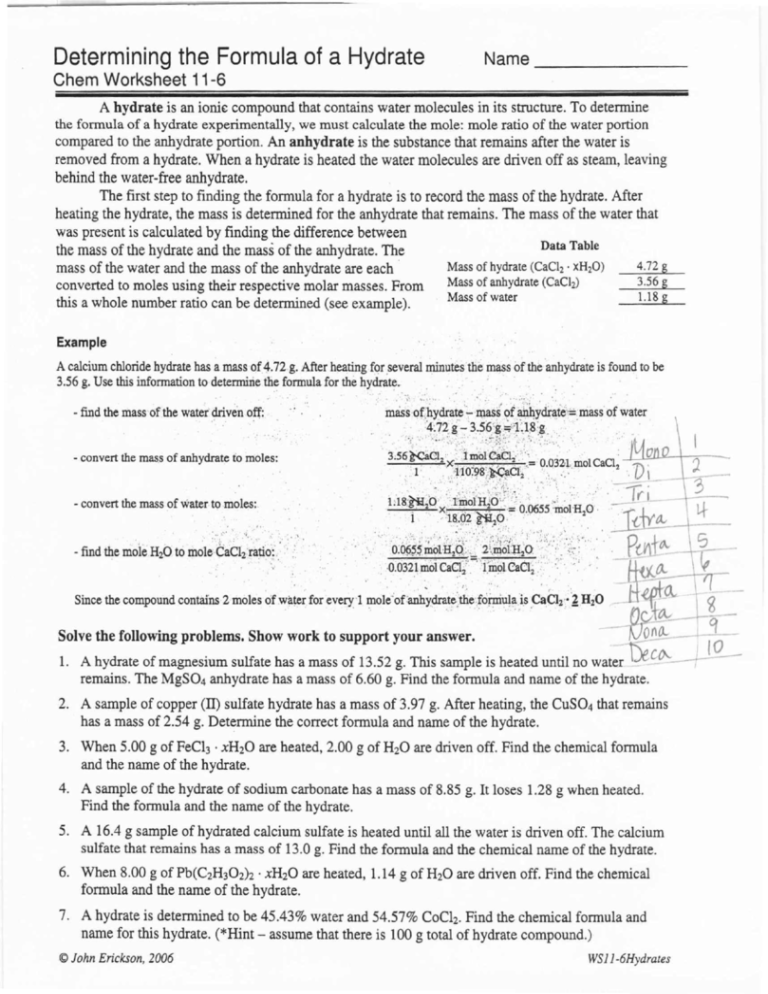
Determining the Formula of a Hydrate
Name
-------------------
Chern Worksheet 11-6
A hydrate is an ionic compound that contains water molecules in its structure. To determine
the formula of a hydrate experimentally, we must calculate the mole: mole ratio of the water portion
compared to the anhydrate portion. An anhydrate is the substance that remains after the water is
removed from a hydrate. When a hydrate is heated the water molecules are driven off as steam, leaving
behind the water-free anhydrate.
The first step to finding the formula for a hydrate is to record the mass of the hydrate. After
heating the hydrate, the mass is determined for the anhydrate that remains. The mass of the water that
was present is calculated by finding the difference between
Data Table
the mass of the hydrate and the mass of the anhydrate. The
4.72g
Mass of hydrate (CaC12 • xH20)
mass of the water and the mass of the anhydrate are each .
3.56 g
Mass
of
anhydrate
(CaCI
)
2
converted to moles using their respective molar masses. From
Mass of water
1.18 g
this a whole number ratio can be determined (see example).
Example
of the anhydrate
A calcium chloride hydrate has a mass of 4.72,g. After heating for several mmutes·the mass
3.56 g. Use this information to determirie the formula for the hydrate.
..;.
..
',-.
"
'
.
....\...
'.
i:nas~:qfhydrate'~
- find the mass-of.the water'driven off:
..
',
)~.
..
.
'.
,'..,
is found to be
.
.
.
,~t 1;>(a$ydi:lI:te= mass' of water
.3,~k.~!~;t~~:~~f,
..: ..D .
9·
"'-','
L" ·J..!Oa'
- convert the mass of anhydrate to moles:
Ul--t-:;::;--
i;l;~,~;··::r:l~~·,
,:_,> .....y:.. ..'
- convert the mass of water to moles:.
. . 1 .:
,2:;e ,'" '. ,';-' z. . y.=
0321..tp.olCael.
~.'i8·(h~6·:,:"·q··Q655·moIH10· ..
' , .
...' '..,or£§;tH,¢J,~;~~~
':::~'~
'i.'~.. .;_JR~~~t:":tA.--r-.
Since the compound contains 2 moles
dr water for everyl
~OI~·Of'anh~e,d.dOiuW"'ls
Solve the following problems. Show work 10 support your answer.
l.
l.l~tA-;,:-__'rn'-
': .O.032.tmolea:~~·. l.mQlcaCl~:.,:····'-: '.
,cai:I/a H,O
-
1:-
-j!fono..~-__.-j~:--;;;:16
A hydrate of magnesium sulfate has a mass of 13.52 g. This sample is heated until no water ~C~~remains. The MgS04 anhydrate has a mass of 6.60 g. Find the formula and name of the hydrate.
2. A sample of copper (II) sulfate hydrate has a mass of 3.97 g. After heating, the CUS04 that remains
has a mass of 2.54 g. Determine the correct formula and name of the hydrate.
3. When 5.00 g of FeCl3 . xH20 are heated, 2.00 g of H20 are driven off. Find the chemical formula
and the name of the hydrate.
4. A sample of the hydrate of sodium carbonate has a mass of 8.85 g. It loses l.28 g when heated.
Find the formula and the name of the hydrate.
5. A 16.4 g sample of hydrated calcium sulfate is heated until all the water is driven off. The calcium
sulfate that remains has a mass of 13.0 g. Find the formula and the chemical name of the hydrate.
6. When 8.00 g of Pb(C2H302)2 . xH20 are heated, 1.14 g of H20 are driven off. Find the chemical
formula and the name of the hydrate.
7. A hydrate is determined to be 45.43% water and 54.57% CoCh. Find the chemical formula and
name for this hydrate. (*Hint - assume that there is 100 g total of hydrate compound.)
© John Erickson, 2006
WSll-6Hydrates
~s
lfaQ
~~
J0~o_
L
M,SOt/
t
1t ~.5Hir()
z-
. r. I _J.
Mo..qMSIUM
M-f)LtS
'i5t:fJLf
L n Aiahy
'~'1--
clr~
I
05LI-g' 3
t; ~~
Mj.5 l/ • 7 HiT 0
J.
~g,S5
31 Cfl ~ t\~dv-olt .
~ IS If ~~
cf a.hhychCLh
4L/ytOO')_
1,L/35
Tsq,&d3/~
3dl 01
H;-O
~I
lf33 !til0
~
I-\J-0
-~
eu.504
I
I"" ( -.
0'1 q";(p ,.._ol H;,<J
IgIOP"
.5
.01q ~~
"
jlAJlLts Hr-0
, yvtAlCv.5°tf
.olsq
p-ente< yel ab
~tJJ)~
SHaO
tuS0'1
3.
r-e_c..IJ·
'(H'JD
5,003 flydrcJ:t
- d ,0 OJ' (,J~r
3,005
3,00
unhydyalt
~C.6
(etb
Inw_tFtClJ I bJ.,Q9PeCl]
O,OI~5~
-
at Fe c'1
_J_
3
::
--J
/
100Q ~
(oqq - -::
to
, o/fr5
!I"D
Ft tl3 ' IoHa D
Iron 8 ortc1 h_u.a.hy dr«lt
q.
Na.~W3'_Hc90
J(p.:1.
IlJ,Di
3(/&,00)
rg.~53 hydro,h
f.J~?J
Hq-O
/05;Q1
1.518 ahh~crr~
I
Scx:{/UYYl ~rhot1dl MonoJlydrafe
0 '71
}J(). d- W3 ' /
,011
s.
I (P. LfJ hydroJ1
3 g,()1
Lf[/vlOo) _
/)(P,j5
1310,15;
*'ifo
~,lj-3Hpl Iw.oL
IIi} 0
'io,o~
13, o~Cct50}:f/lhtot
-13. 05 ~hnvc{(~
3·4-5
,qqj
~,1'(~1
I <6, O~J
• !«
81~
H,:p
;. d tllO/ tla- v
• DqSL{~~WOlf
1~;;:O'l
~a.1CA um SuI nu
Ca SOLf ,,~HJ- 0
dOl,~
4LI s.o ,)
ltL/IDt)
~ [/1,,00)
3as3
,0('33
~
_ ~
-
1,..vYl Pb(eJU30~\
\>\ ~W\\oQlA.5 (lc.,vlcde. -\Yi h'fdy~
"¥bLtd \i30'_\~• 3 Ha-0
D/hyd~fe
~ 5 .4 3 10 H iJo
54,511 tt>C.A~
'15 ,I-IJ,j H!!_-O
I m.otlf;-'O -:;.. J, 5 ~ II ~
If,O{)j H.,D
HI' 0
I • & If
Co t J
. Q-
0








