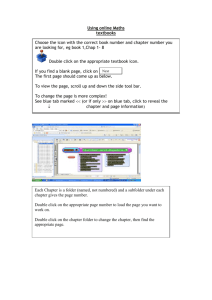
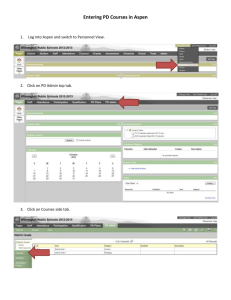
Complete Volume Thirteen 2013
advertisement

FEBRUARY 2013 • VOLUME 13-01 PHARMACISTS’ EDITION Nova Scotia Formulary Updates Nova Scotia Formulary Updates Prescriber Validation www.medavie.bluecross.ca\healthprofessionals PAGE 2 OF 5 PHARMACISTS’ EDITION VOLUME 13-01 Criteria Update – Botox® (200u/vial Inj) February 1, 2013 PRODUCT STRENGTH DIN PRESCRIBER BENEFIT STATUS MFR PRESCRIBER BENEFIT STATUS MFR Botox® (onabotulinumtoxin A) Criteria Update – Actemra® (Tocilizumab) February 1, 2013 PRODUCT Actemra® (tocilizumab) STRENGTH DIN PAGE 3 OF 5 PHARMACISTS’ EDITION VOLUME 13-01 Insured Pediatric Compounded Solutions New Diabetic Products February 1, 2013 PRODUCT DIN/PIN New Line of Ostomy Products February 1, 2013 PRODUCT NUMBER PRESCRIBER BENEFIT STATUS MFR PAGE 4 OF 5 PHARMACISTS’ EDITION VOLUME 13-01 Non-Insured Products PRODUCT STRENGTH DIN PRESCRIBER BENEFIT STATUS MFR STRENGTH DIN PRESCRIBER BENEFIT STATUS MFR Rapaflo® (sildosin) PRODUCT Targin® (naloxone/oxycodone) Standardization of Package Sizes FORM QUANTITY FORM QUANTITY PAGE 5 OF 5 PHARMACISTS’ EDITION VOLUME 13-01 Standardization of Package Sizes Continued… FORM QUANTITY Appendix I Alternate Prescriber Numbers The following physicians previously had alternate billing numbers. As of Feb 15, 2013 please use their respective College of Physicians and Surgeons of Nova Scotia (CPSNS) license numbers when billing claims to Nova Scotia Pharmacare. SURNAME TOWN/CITY SURNAME TOWN/CITY AGO, C ALLAN, PATRICIA ARCHIBALD, JOHN BARRY, ANNE BETHUNE, GRAEME BOWIE, DENNIS BUCHHOLZ, KENNETH CHEEVERS, PAUL CLARK, ALEXANDER COMEAU, ALBAN COOPER, ROBERT CRASWELL, DONALD DAS, BIJON DAVIS, HEATHER DESROSIERS, JACQUES DEVITT, JAMES DICKINSON, JOHN ELLIOTT, CHRISTOPHER FELTMATE, MARY GHENEA, IRINA GILLIS, GRANT GUPTILL, JONI HAMILTON, JOHN HANADA, EDWIN HEGARTY, RAYMOND HIMMELMAN, DONALD HORREY, KATHLEEN HUMAYUN, MUHAMMAD JARVIS, CARL JHA, UMESH JOHNSTON, CHRISTOPHER JOLLYMORE, GEORGE JOST, ELLEN KHALIL, HISHAM KIRBY, RONALD KNIGHT, DEBORAH LANGILLE, KENNETH LUDMAN, MARK MACDONALD, KAREN MACDONALD, KAREN MACGIBBON, S LOIS MACINTOSH, DONALD HALIFAX ANTIGONISH SYDNEY KENTVILLE HALIFAX HALIFAX ANNAPOLIS ROYAL YARMOUTH HALIFAX SAULNIERVILLE PICTOU MIDDLETON HALIFAX KENTVILLE HALIFAX HAMMONDS PLAINS HALIFAX NEW GLASGOW TRURO NORTH SYDNEY HALIFAX DARTMOUTH ANTIGONISH HALIFAX ANTIGONISH PLEASANTVILLE HALIFAX DARTMOUTH HALIFAX HALIFAX FALL RIVER CHESTER HALIFAX BEDFORD HALIFAX DARTMOUTH AYLESFORD HALIFAX ANTIGONISH HALIFAX BEDFORD HALIFAX MACINTOSH, REBECCA MACKAY, THOMAS MACKNIGHT, CHRIS MACNEIL, MARY MARSH, LORNE MCGIBNEY, KIERON MCNEILL, LAURIE MILNE, P RONALD MORSE, DAVID MORSE, EWART NADER, NABIL O'BRIEN, BRIAN ORLIK, BENJAMIN PEARCE, PATRICIA PESTELL, DEBBIE REID, DANIEL RICHARDSON, C GLEN RIIVES, MAI ROGERS, JOHN RONDEAU, RONALD ROY, GREGORY SAWLER, MARGARET SCHAFFNER, JOHN SCOTT, TRACY SLAYTER, IAN SMITH, CHERYL SMITH, MURDOCK SMITH, PETER STACEY, COOPER STEVENS, SARAH TRITES, JONATHAN WAWER, ANDREW WAWER, URSULA WERTLEN, WINSTON WOOD, WILLIAM HALIFAX HALIFAX HALIFAX HALIFAX HALIFAX TRURO BRIDGEWATER HALIFAX LUNENBURG BRIDGEWATER AMHERST HALIFAX HALIFAX HALIFAX HALIFAX DARTMOUTH HALIFAX HALIFAX SYDNEY OXFORD DARTMOUTH WAVERLEY PORT WILLIAMS HALIFAX ANTIGONISH SCOTSBURN SYDNEY DARTMOUTH DARTMOUTH HALIFAX HALIFAX NORTH SYDNEY BEDFORD YARMOUTH BEDFORD FEBRUARY 2013 Appendix II Insured Pediatric Compounded Solutions COMPOUND acetazolamide oral suspension PIN 00903403 COMPOUND nitrazepam oral suspension PIN 00903215 allopurinol oral suspension 00903171 nitrofurantoin oral suspension 00903209 amiodarone oral suspension 00903325 propranolol oral suspension 00999155 amlodipine oral suspension 00903749 pyrazinamide oral suspension 00903781 atenolol oral syrup 00903346 sotalol oral suspension 00903782 azathioprine oral suspension 00903187 spironolactone oral suspension 00999107 baclofen oral suspension 00903511 sulfasalazine oral suspension 00903449 carvedilol oral suspension 00903641 verapamil oral suspension 00903009 clonazepam oral suspension 00903559 clonidine oral suspension 00999330 clotrimazole oral suspension 00903061 dexamethasone oral suspension 00903062 domperidone oral suspension 00903085 enalapril oral suspension 00903554 hydralazine oral suspension 00903591 hydrochlorothiazide oral suspension 00999106 hydrocortisone oral suspension 00903296 indomethacin oral suspension 00903250 labetolol oral suspension 00903077 lamotrigine oral suspension 00903381 lansoprazole oral suspension 00903192 lisinopril oral suspension 00903266 methimazole oral suspension 00903779 metolazone oral suspension 00903780 metoprolol oral suspension 00999104 metronidazole oral suspension 00903238 nadolol oral syrup 00903406 naproxen oral suspension 00999135 FEBRUARY 2013 MARCH 2013 • VOLUME 13-02 PHARMACISTS’ EDITION Nova Scotia Formulary Updates Nova Scotia Formulary Updates Pan-Canadian Generic Price Initiative – Changes to Maximum Reimbursable Price (MRP) Pan-Canadian Generic Price Initiative - Changes to Maximum Reimbursable Price (MRP) New Exception Status Benefits - Zelboraf® Sensipar® Toviaz® Criteria Update - Rituxan® Tasigna® Update to Reimbursement List PRP Reminder about Prescription Adaptation Eligibility On January 18, 2013 the Council of the Federation announced the pan-Canadian Competitive Value Price Initiative for Generic Drugs. Nova Scotia participated in this initiative along with other provinces and territories. Further information on the announcement is available at http://www.councilofthefederation.ca/pdfs/NR-CoFGeneric%20drugs%20(Final)-Jan%2018.pdf. The confirmation of the pricing for this initiative is now complete and includes the following interchangeable categories. Note that the prices will change effective April 1, 2013 and the new MRP will be effective April 1, 2013. A copy of the April Reimbursement List can be found with the electronic copy of the bulletin at http://novascotia.ca/health/Pharmacare/info_pro/pharmacare-news.asp. PRODUCT NEW MRP amlodipine 5mg tab 0.2417 amlodipine 10mg tab 0.3587 atorvastatin 10mg tab 0.3138 atorvastatin 20mg tab 0.3922 atorvastatin 40mg tab 0.4216 atorvastatin 80mg tab 0.4216 omeprazole 20mg cap 0.4117 rabeprazole 10mg EC tab 0.1204 rabeprazole 20mg EC tab 0.2408 PAGE 2 OF 6 PHARMACISTS’ EDITION VOLUME 13-02 Pan-Canadian Generic Price Initiative Continued… PRODUCT NEW MRP ramipril 1.25mg cap 0.1274 ramipril 2.5mg cap 0.1470 ramipril 5mg cap 0.1470 ramipril 10mg cap 0.1862 venlafaxine 37.5mg ER cap 0.1643 venlafaxine 75mg ER cap 0.3285 venlafaxine 150mg ER cap 0.3469 New Exception Status Benefits The following product was reviewed by the pCODR expert advisory committee (pERC) and will be listed as an exception status benefit, with the following criteria, effective March 4, 2013. PRODUCT STRENGTH DIN PRESCRIBER Zelboraf® (vemurafenib) 240mg tablet 02380242 DNP Criteria • • Decision Highlights • • BENEFIT STATUS E MFR HLR as a first line, single agent for the treatment of BRAF V600 mutation positive unresectable or metastatic melanoma in patients with an ECOG performance status (PS) of ≤ 1 for BRAF V600 mutation positive patients who have progressed after first line treatment prior to vemurafenib availability, funding of vemurafenib as a second line agent may be considered One open-label randomized controlled trial compared vemurafenib with dacarbazine in previously untreated patients with unresectable stage IIIC or IV melanoma who were positive for the BRAF V600 mutation. This study showed a net clinical benefit with vemurafenib therapy versus dacarbazine. Approved dosage of vemurafenib is 960mg (4x240mg tablets) twice daily, continued until disease progression. A Pharmacare reimbursement price of $50.4980 per tablet has been assigned. Also please note, if the claim exceeds a value of $9,999.99, the claim must be divided and processed as two separate transactions: • The first transaction should be submitted using the DIN 02380242 and the quantity should be adjusted so the total claim (including the ingredient cost, professional fee, and markup) does not exceed $9,999.99. This claim will allow markup to the $250 maximum. • The second transaction should be submitted with the remaining quantity using the PIN 00903786. This PIN will only pay ingredient cost. • The copay and deductible will be applied to both claims for beneficiaries enrolled in the Seniors’ and Family Pharmacare Programs. PAGE 3 OF 6 PHARMACISTS’ EDITION VOLUME 13-02 New Exception Status Benefits Continued… The following product will be listed as an exception status benefit, with the following criteria, effective March 4, 2013. PRODUCT STRENGTH DIN PRESCRIBER Sensipar® (cinacalcet) 30mg 60mg 90mg 02257130 02257149 02257157 DNP DNP DNP Criteria • BENEFIT STATUS E E E MFR AGA AGA AGA For the treatment of patients with chronic kidney disease on dialysis with severe secondary hyperparathyroidism who - are not responding to optimal doses of Vitamin D analogues or phosphate binders (calcium or non-calcium based) AND are either not a surgical candidate due to surgical or anesthetic risk OR awaiting kidney transplant - in addition laboratory findings must confirm serum phosphate >1.8mmol/L, serum calcium ≥2.2mmol/L and iPTH >88pmol/L on more than one occasion at least 6 weeks apart - ongoing laboratory investigations must include serum calcium, albumin, phosphorous weekly for three weeks and iPTH every 6 weeks - coverage for cinacalcet will be renewed if there is a greater than 30% decrease in iPTH after at least 3 months with escalating dose, indicating the patient is responding - approval period 12 months, provided there has been a greater than 30% decrease in iPTH as stated above The following product was reviewed by the Canadian Drug Expert Committee (CDEC) and will be listed as an exception status benefit, with the following criteria, effective March 4, 2013. BENEFIT MFR STATUS Toviaz® 4mg tab 02380021 DNP E PFI (fesoterodine fumerate) 8mg tab 02380048 DNP E PFI Criteria • for the treatment of over-active bladder (not stress incontinence) for patients who cannot tolerate immediate release oxybutynin after an adequate trial (e.g. 3 months) • a three month trial will be approved initially with assessment of the effectiveness of this therapy required if further coverage is considered Decision Highlights • In three double-blind, randomized controlled trials in patients with overactive bladder, fesoterodine produced similar reductions in daily urinary urge incontinence and micturition events as sustained release tolterodine. PRODUCT STRENGTH DIN PRESCRIBER PAGE 4 OF 6 PHARMACISTS’ EDITION VOLUME 13-02 Criteria Update – Rituxan® Please note that effective March 4, 2013, the criteria for Rituxan will be updated to include the following: PRODUCT STRENGTH DIN PRESCRIBER Rituxan® (rituximab) 10mg/mL 02241927 DNP Criteria • Decision Highlights • • • • BENEFIT STATUS E MFR HLR for the induction of remission in patients with severely active granulomatosis with polyangitis (GPA) or microscopic polyangitis (MPA) who have severe intolerance or other contraindication to cyclophosphamide, or who have failed an adequate trial of cyclophosphamide GPA (also known as Wegner’s Granulomatosis) and MPA are the two major forms of systemic vasculitis associated with the presense of anti-neutrophil cytoplasmic antibodies (ANCAs). The pro-inflammatory effects of ANCA produce endothelial injury and tissue damage. In one double-blind RCT, rituximab was reported to be non-inferior, but not superior, to oral cyclophosphamide for inducing remission in patients with severely active GPA or MPA, based on the number of patients who achieved complete remission at six months. The approved dose is 375mg/m2 as an IV infusion once weekly for four weeks. The committee considered an adequate trial of cyclophosphamide to be six IV pulses or 3 months of oral therapy. Criteria Update – Tasigna® Please note that effective March 4, 2013, the criteria for Tasigna will be updated to the following: PRODUCT Tasigna® (nilotinib) STRENGTH DIN PRESCRIBER BENEFIT STATUS E E MFR 150mg cap 02368250 DNP NVR 200mg cap 02315874 DNP NVR Criteria First Line: • As a single first line agent for the treatment of adults with chronic phase CML Second Line: • As a single second line agent for the treatment of adults with chronic or accelerated phase CML with resistance or intolerance to prior therapy These second line criteria include: 1. Patients with CML in chronic phase who are intolerant to oral tyrosine kinase inhibitors (TKIs) (i.e. imatinib or dasatinib or both) 2. Patients with CML in chronic phase who are resistant to imatinib 3. Patients with CML that have progressed to accelerated phase while on imatinib therapy • In any one patient, only two of the TKIs will be funded within these criteria during their lifetime • If a patient develops grade 3 or 4 toxicity to one of the TKIs used within 3 months of initiating therapy, access to a third agent will be funded • Sequential use of nilotinib and dasatinib is not permitted except in the circumstance described above (i.e. grade 3 or 4 toxicity) PAGE 5 OF 6 PHARMACISTS’ EDITION VOLUME 13-02 Update to Reimbursement List PRP The following products will have Pharmacare Reimbursement prices, as noted below, effective March 4, 2013. PRODUCT PRP omeprazole 10mg cap 0.2059 vemurafenib (Zelboraf) 240mg tab 50.4980 PAGE 6 OF 6 PHARMACISTS’ EDITION VOLUME 13-02 Reminder about Prescription Adaptation Eligibility Prescription adaptation (PA) is an insured service under all the Pharmacare Programs when it is performed as follows: 1. Refusal to fill a drug monitored by the Prescription Monitoring Program. 2. For a clinical reason to enhance patient outcomes such as dose, duration, adverse drug reaction, or intolerance. Note: Changes in prescription quantity not related to a dose or duration change or changes in formulation are not insured PA services. To qualify for the program: • • • The individual must be a beneficiary of a Nova Scotia Pharmacare Program. The beneficiary must give informed and voluntary consent as described in the Nova Scotia College of Pharmacist Standards of Practice for Prescribing Drugs by Pharmacists. Pharmacists must comply with all applicable Nova Scotia College of Pharmacists, Standards of Practice for Prescribing Drugs by Pharmacists. Documentation of consent, assessment, monitoring plan and notification to the prescriber of the medication that being adapted is to be kept on file in the pharmacy for at least three years for audit purposes. Pharmacists must submit an adverse drug reaction (ADR) report if the adaptation is done for a clinical reason such as an adverse drug reaction or intolerance to a drug. A copy of the Health Canada ADR report is to be kept on file in the pharmacy for at least three years for audit purposes. All information on the documentation required can be found under Nova Scotia College of Pharmacists, Standards of Practice for Prescribing Drugs by Pharmacists. Forms can be found on the Nova Scotia College of Pharmacists website or the Pharmacy Association of Nova Scotia website. Helpful reminders: • • • • • Changes in prescription quantity not related to a dose or duration change or changes in formulation are not insured PA services. Any changes required for compliance packaging must be authorized by the original prescriber. Changes made to match the quantity prescribed to a commercially available package size are also not eligible. Prescription adaptations are not paid for substituting another strength in the case of a manufacturer shortage (e.g., Synthroid® 0.2mg changed to 2 x Synthroid® 0.1mg) In the case where the prescriber has written a prescription for a drug and/or strength that does not exist, pharmacists cannot follow the two step claims process outlined in the Pharmacists’ Guide. In these cases they can bill for the adaptation, then process the adapted prescription and ensure the situation is documented clearly. Refusal to fill is only reimbursed for drugs monitored by the Prescription Monitoring Program, no other drugs are eligible under for this service (e.g., diazepam, clonazepam, cyclobenzaprine) Refusal to fill is only paid when in the pharmacist’s professional judgment, a prescription is falsified or adulterated, when there is suspected multi-pharmacy, multi doctoring, or there is potential for overuse or abuse. Refusing to fill a part fill earlier than indicated on the original prescription by the prescriber is not eligible. NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 1 Generic Name and Strength abiraterone 250mg cap (exception status) DIN 02371065 Zytiga 250mg cap MFR MRP JAN acebutolol HCl 100mg tab 02286246 02147602 02237885 02237721 02204517 01926543 02286254 02147610 02237886 02237722 02204525 01926551 Acebutolol 100mg tab Apo-Acebutolol 100mg tab MYLAN-Acebutolol (Type S) 100mg tab MYLAN-Acebutolol 100mg tab Novo-Acebutolol 100mg tab Sectral 100mg tab Acebutolol 200mg tab Apo-Acebutolol 200mg tab MYLAN-Acebutolol (Type S) 200mg tab MYLAN-Acebutolol 200mg tab Novo-Acebutolol 200mg tab Sectral 200mg tab SAS APX MYL MYL TEV SAV SAS APX MYL MYL TEV SAV 0.1175 0.1175 0.1175 0.1175 0.1175 0.1175 0.1762 0.1762 0.1762 0.1762 0.1762 0.1762 acebutolol HCl 400mg tab 02286262 02147629 02237887 02237723 02204533 01926578 Acebutolol 400mg tab Apo-Acebutolol 400mg tab MYLAN-Acebutolol (Type S) 400mg tab MYLAN-Acebutolol 400mg tab Novo-Acebutolol 400mg tab Sectral 400mg tab SAS APX MYL MYL TEV SAV 0.3507 0.3507 0.3507 0.3507 0.3507 0.3507 acetaminophen 325mg & oxycodone 5mg tab 02324628 Apo-Oxycodone/Acet 5/325mg tab APX 0.1285 01916548 02361361 01916475 00608165 02307898 Endocet tab Oxycodone/Acet 5/325mg tab Percocet tab ratio-Oxycocet tab Sandoz-Oxycodone Acet tab BRI SAS BRI TEV SDZ 0.1285 0.1285 0.1285 0.1285 0.1285 00545015 Acetazolamide tablets 250mg AAP 0.1343 02243098 02091526 00010332 00216666 02284529 00010340 00229296 00176192 Acetylcysteine 200mg/mL inj Mucomyst 200mg/mL inj Entrophen 325mg EC tab Novasen 325mg EC tab pms-ASA 325mg EC tab Entrophen 650mg EC tab Novasen 650mg EC tab Fiorinal C1/4 cap SDZ WLS PDP TEV PMS PDP TEV NVR 0.6800 0.6800 0.0280 0.0280 0.0280 0.0352 0.0352 0.6446 00608203 00176206 ratio-Tecnal C1/4 cap Fiorinal C1/2 cap TEV NVR 0.6446 0.7896 00608181 00226327 ratio-Tecnal C1/2 cap Fiorinal cap TEV NVR 0.7896 0.6014 00608238 02286556 ratio-Tecnal cap Acyclovir 200mg tab TEV SAS 0.6014 0.6397 acebutolol HCl 200mg tab acetazolamide 250mg tab acetylcysteine 200mg/mL inj acetylsalicylic acid 325mg EC tab acetylsalicylic acid 650mg EC tab acetylsalicylic acid 330mg, butalbital 50mg, caffeine 40mg & codeine phosphate 15mg cap acetylsalicylic acid 330mg, butalbital 50mg, caffeine 40mg & codeine phosphate 30mg cap acetylsalicylic acid 330mg, butalbital 50mg & caffeine 40mg cap acyclovir 200mg tab Brand Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 1 of 87 2 PRP 30.7417 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength acyclovir 200mg tab DIN 02207621 02242784 02285959 02078627 00634506 Brand Apo-Acyclovir 200mg tab MYLAN-Acyclovir 200mg tab Novo-Acyclovir 200mg tab ratio-Acyclovir 200mg tab Zovirax 200mg tab MFR MRP APX 0.6397 MYL 0.6397 TEV 0.6397 TEV 0.6397 GSK 0.6397 acyclovir 400mg tab 02286564 02207648 02242463 02285967 02078635 01911627 Acyclovir 400mg tab Apo-Acyclovir 400mg tab MYLAN-Acyclovir 400mg tab Novo-Acyclovir 400mg tab ratio-Acyclovir 400mg tab Zovirax 400mg tab SAS APX MYL TEV TEV GSK 1.2700 1.2700 1.2700 1.2700 1.2700 1.2700 acyclovir 800mg tab 02286572 02207656 02242464 02285975 02078651 Acyclovir 800mg tab Apo-Acyclovir 800mg tab MYLAN-Acyclovir 800mg tab Novo-Acyclovir 800mg tab ratio-Acyclovir 800mg tab SAS APX MYL TEV TEV 1.7742 1.7742 1.7742 1.7742 1.7742 adalimumab 50mg/mL inj (exception status) 02258595 Humira 40mg/0.8mL inj ABB 989.2813 97799756 97799757 02248728 02201011 02270129 02384701 02288087 02247373 Humira 40mg/0.8mL pen Humira 40mg/0.8mL syringe inj Apo-Alendronate 10mg tab Fosamax 10mg tab (discontinued) MYLAN-Alendronate 10mg tab RAN-Alendronate 10mg tab Sandoz Alendronate 10mg tab Teva-Alendronate 10mg tab ABB ABB APX FRS MYL RAN SDZ TEV 989.2813 989.2813 0.6981 0.6981 0.6981 0.6981 0.6981 0.6981 alendronate 40mg tab (exception status) 02258102 02201038 CO Alendronate 40mg tab Fosamax 40mg tab COB FRS 3.0557 3.0557 alendronate 70mg tab (exception status) 02352966 02299712 02248730 02258110 02245329 02385031 02286335 02284006 02384728 02288109 02261715 00402818 Alendronate 70mg tab Alendronate-FC 70mg tab Apo-Alendronate 70mg tab CO Alendronate 70mg tab Fosamax 70mg tab Jamp-Alendronate 70mg tab MYLAN-Alendronate 70mg tab pms-Alendronate-FC 70mg tab RAN-Alendronate 70mg tab Sandoz Alendronate 70mg tab Teva-Alendronate 70mg tab Zyloprim 100mg tab SAS PHL APX COB FRS JPC MYL PMS RAN SDZ TEV AAP 3.5201 3.5201 3.5201 3.5201 3.5201 3.5201 3.5201 3.5201 3.5201 3.5201 3.5201 0.0846 00479799 00402796 02349191 00865397 02137534 Zyloprim 200mg tab Zyloprim 300mg tab Alprazolam 0.25mg tab Apo-Alpraz 0.25mg tab MYLAN-Alprazolam 0.25mg tab AAP AAP SAS APX MYL 0.1411 0.2306 0.0760 0.0760 0.0760 alendronate 10mg tab (exception status) allopurinol 100mg tab allopurinol 200mg tab allopurinol 300mg tab alprazolam 0.25mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 2 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength alprazolam 0.25mg tab DIN 01913484 00548359 Brand Teva-Alprazolam 0.25mg tab Xanax 0.25mg tab MFR MRP TEV 0.0760 PFI 0.0760 alprazolam 0.5mg tab 02349205 00865400 02137542 01913492 00548367 02139200 01990403 Alprazolam 0.5mg tab Apo-Alpraz 0.5mg tab MYLAN-Alprazolam 0.5mg tab Teva-Alprazolam 0.5mg tab Xanax 0.5mg tab MYLAN-Amantadine 100mg cap pms-Amantadine 100mg cap SAS APX MYL TEV PFI MYL PMS 0.0920 0.0920 0.0920 0.0920 0.0920 0.5179 0.5179 amantadine HCl 10mg/mL o/l amcinonide 0.1% cr 02022826 02192284 02247098 02246714 pms-Amantadine 10mg/mL syrup Cyclocort 0.1% cr ratio-Amcinonide 0.1% cr Taro-Amcinonide 0.1% cr PMS STI TEV TAR 0.1005 0.1953 0.1953 0.1953 amcinonide 0.1% lot 02192276 02247097 02192268 02247096 02249510 01997580 02171929 Cyclocort 0.1% lot ratio-Amcinonide 0.1% lot Cyclocort 0.1% oint ratio-Amcinonide 0.1% oint Midamor 5mg tab Asacol 400mg tab Novo-5-ASA 400mg EC tab STI TEV STI TEV AAP WNC TEV 0.2714 0.2714 0.3776 0.3776 0.2948 0.4039 0.4039 amitriptyline 10mg tab 02364336 02246194 02036282 02240604 02245781 02242472 02240071 02243836 02239835 00335053 Amiodarone 200mg tab Apo-Amiodarone 200mg tab Cordarone 200mg tab MYLAN-Amiodarone 200mg tab phl-Amiodarone 200mg tab pms-Amiodarone 200mg tab ratio-Amiodarone 200mg tab (discontinued) Sandoz Amiodarone 200mg tab Teva-Amiodarone 200mg tab Elavil 10mg tab SAS APX WAY MYL PHL PMS TEV SDZ TEV AAP 0.7206 0.7206 0.7206 0.7206 0.7206 0.7206 0.7206 0.7206 0.7206 0.0721 amitriptyline 25mg tab 00335061 Elavil 25mg tab AAP 0.1314 amitriptyline 50mg tab 00335088 Elavil 50mg tab AAP 0.2547 amitriptyline 75mg tab amlodipine 5mg tab 00754129 02331284 02378760 02273373 02297485 02280132 02331071 02357194 02371715 02362651 02272113 00878928 Elavil 75mg tab Amlodipine 5mg tab Amlodipine-ODAN 5mg tab Apo-Amlodipine 5mg tab CO Amlodipine 5mg tab GD-Amlodipine 5mg tab Jamp-Amlodipine 5mg tab Jamp-Amlodipine 5mg tab Mar-Amlodipine 5mg tab MINT-Amlodipine 5mg tab MYLAN-Amlodipine 5mg tab Norvasc 5mg tab AAP SAS ODN APX COB GMD JPC JPC MAR MNT MYL PFI 0.3943 0.2417 0.2417 0.2417 0.2417 0.2417 0.2417 0.2417 0.2417 0.2417 0.2417 0.2417 amantadine HCl 100mg cap amcinonide 0.1% oint amiloride 5mg tab 5-aminosalicylic acid 400mg tab amiodarone 200mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 3 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength amlodipine 5mg tab DIN 02326779 02284065 02321858 02259605 02284383 02357712 02250497 Brand phl-Amlodipine 5mg tab pms-Amlodipine 5mg tab RAN-Amlodipine 5mg tab ratio-Amlodipine 5mg tab Sandoz Amlodipine 5mg tab Septa-Amlodipine 5mg tab Teva-Amlodipine 5mg tab MFR MRP PHL 0.2417 PMS 0.2417 RAN 0.2417 TEV 0.2417 SDZ 0.2417 SPT 0.2417 TEV 0.2417 amlodipine 10mg tab 02331292 02378779 02273381 02297493 02280140 02331098 02357208 02371723 02362678 02272121 00878936 02326787 02284073 02321866 02259613 02284391 02357720 02250500 Amlodipine 10mg tab Amlodipine-ODAN 10mg tab Apo-Amlodipine 10mg tab CO Amlodipine 10mg tab GD-Amlodipine 10mg tab Jamp-Amlodipine 10mg tab Jamp-Amlodipine 10mg tab Mar-Amlodipine 10mg tab MINT-Amlodipine 10mg tab MYLAN-Amlodipine 10mg tab Norvasc 10mg tab phl-Amlodipine 10mg tab pms-Amlodipine 10mg tab RAN-Amlodipine 10mg tab ratio-Amlodipine 10mg tab Sandoz Amlodipine 10mg tab Septa-Amlodipine 10mg tab Teva-Amlodipine 10mg tab SAS ODN APX COB GMD JPC JPC MAR MNT MYL PFI PHL PMS RAN TEV SDZ SPT TEV 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 0.3587 amlodipine 5mg & atorvastatin 10mg tab 02273233 02362759 02273241 02362767 Caduet 5/10mg tab GD-Amlodipine/Atorvastatin 5/10mg tab Caduet 5/20mg tab GD-Amlodipine/Atorvastatin 5/20mg tab PFI GMD PFI GMD 1.0310 1.0310 1.1766 1.1766 02273268 02362775 02273276 02362783 02273284 02362791 02273292 02362805 02273306 02362813 Caduet 5/40mg tab GD-Amlodipine/Atorvastatin 5/40mg tab Caduet 5/80mg tab GD-Amlodipine/Atorvastatin 5/80mg tab Caduet 10/10mg tab GD-Amlodipine/Atorvastatin 10/10mg tab Caduet 10/20mg tab GD-Amlodipine/Atorvastatin 10/20mg tab Caduet 10/40mg tab GD-Amlodipine/Atorvastatin 10/40mg tab PFI GMD PFI GMD PFI GMD PFI GMD PFI GMD 1.2312 1.2312 1.2312 1.2312 1.2483 1.2483 1.3939 1.3939 1.4485 1.4485 02273314 02362821 02243986 Caduet 10/80mg tab GD-Amlodipine/Atorvastatin 10/80mg tab Apo-Amoxi Clav 125mg/5mL susp PFI GMD APX 1.4485 1.4485 0.0517 01916882 02244646 Clavulin-125F 125mg/5mL susp ratio-Aclavulanate 125mg/5mL susp GSK TEV 0.0517 0.0517 amlodipine 5mg & atorvastatin 20mg tab amlodipine 5mg & atorvastatin 40mg tab amlodipine 5mg & atorvastatin 80mg tab amlodipine 10mg & atorvastatin 10mg tab amlodipine 10mg & atorvastatin 20mg tab amlodipine 10mg & atorvastatin 40mg tab amlodipine 10mg & atorvastatin 80mg tab amoxicillin & enzyme inhibitor 125mg/5mL susp Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 4 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength amoxicillin & enzyme inhibitor 250mg/5mL susp DIN 02243987 Brand Apo-Amoxi Clav 250mg/5mL susp MFR MRP APX 0.0869 01916874 02244647 02288559 Clavulin-250F 250mg/5mL susp ratio-Aclavulanate 250mg/5mL susp Apo-Amoxi Clav 400mg/5mL susp GSK TEV APX 0.0869 0.0869 0.1969 02238830 02243350 Clavulin-400 400mg/5mL susp Apo-Amoxi Clav 250mg tab GSK APX 0.1969 0.9375 02243351 01916858 02243771 02245623 02238829 02248138 02247021 Apo-Amoxi Clav 500mg tab Clavulin-500F 500mg tab ratio-Aclavulanate 500mg tab Apo-Amoxi Clav 875mg tab Clavulin-875 (875mg) tab Novo-Clavamoxin-875 (875mg) tab ratio-Aclavulanate 875mg tab APX GSK TEV APX GSK TEV TEV 0.6673 0.6673 0.6673 0.7771 0.7771 0.7771 0.7771 amoxicillin 250mg cap 02352710 00628115 02238171 00406724 02230243 Amoxicillin 250mg cap Apo-Amoxi 250mg cap MYLAN-Amoxicillin 250mg cap Novamoxin 250mg cap pms-Amoxicillin 250mg cap SAS APX MYL TEV PMS 0.1750 0.1750 0.1750 0.1750 0.1750 amoxicillin 500mg cap 02352729 00628123 02238172 00406716 02230244 02036355 Amoxicillin 500mg cap Apo-Amoxi 500mg cap MYLAN-Amoxicillin 500mg cap Novamoxin 500mg cap pms-Amoxicillin 500mg cap Novamoxin 250mg chew tab SAS APX MYL TEV PMS TEV 0.3417 0.3417 0.3417 0.3417 0.3417 0.6156 02352745 02352761 00628131 00628131 00452149 01934171 02230245 Amoxicillin 125mg susp Amoxicillin Sugar-Reduced 25mg/mL o/l Apo-Amoxi 25mg/mL o/l Apo-Amoxi Sugar Free 25mg/mL o/l Novamoxin 25mg/mL o/l Novamoxin Sugar-Reduced 25mg/mL o/l pms-Amoxicillin 25mg/mL o/l SAS SAS APX APX TEV TEV PMS 0.0353 0.0353 0.0353 0.0353 0.0353 0.0353 0.0353 02352753 02352788 00628158 00628158 00452130 01934163 02230246 00020877 00020885 02236859 02253054 02260107 Amoxicillin 250mg susp Amoxicillin Sugar-Reduced 50mg/mL o/l Apo-Amoxi 50mg/mL o/l Apo-Amoxi Sugar Free 50mg/mL o/l Novamoxin 50mg/mL o/l Novamoxin Sugar-Reduced 50mg/mL o/l pms-Amoxicillin 50mg/mL o/l Novo-Ampicillin 250mg cap Novo-Ampicillin 500mg cap Agrylin 0.5mg cap MYLAN-Anagrelide 0.5mg cap Sandoz Anagrelide 0.5mg cap SAS SAS APX APX TEV TEV PMS TEV TEV SHI MYL SDZ 0.0540 0.0540 0.0540 0.0540 0.0540 0.0540 0.0540 0.3796 0.7091 2.6361 2.6361 2.6361 amoxicillin & enzyme inhibitor 400mg/5mL susp amoxicillin & enzyme inhibitor 250mg tab amoxicillin & enzyme inhibitor 500mg tab amoxicillin & enzyme inhibitor 875mg tab amoxicillin 250mg chewable tab amoxicillin 25mg/mL o/l amoxicillin 50mg/mL o/l ampicillin 250mg cap ampicillin 500mg cap anagrelide 0.5mg cap (exception status) Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 5 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength anastrozole 1mg tab atenolol 25mg tab atenolol 50mg tab atenolol 100mg tab atenolol 50mg & chlorthalidone 25mg tab DIN 02374420 02224135 02394898 02339080 02379562 02379104 02393573 02361418 02320738 02328690 02338467 02365650 02313049 02247182 02246581 Brand Apo-Anastrozole 1mg tab Arimidex 1mg tab CO Anastrozole 1mg tab Jamp-Anastrozole 1mg tab Mar-Anastrozole 1mg tab MED-Anastrozole 1mg tab MINT-Anastrozole 1mg tab MYLAN-Anastrozole 1mg tab pms-Anastrozole 1mg tab RAN-Anastrozole 1mg tab Sandoz Anastrozole 1mg tab Taro-Anastrozole 1mg tab Teva-Anastrozole 1mg tab phl-Atenolol 25mg tab pms-Atenolol 25mg tab MFR MRP APX 1.7821 AZE 1.7821 COB 1.7821 JPC 1.7821 MAR 1.7821 GMP 1.7821 MNT 1.7821 MYL 1.7821 PMS 1.7821 RAN 1.7821 SDZ 1.7821 TAR 1.7821 TEV 1.7821 PHL 0.0946 PMS 0.0946 00773689 02255545 02367564 02371987 02368021 02146894 02238316 02237600 02267985 02171791 02231731 02368641 02039532 00773697 02255553 02367572 02371995 02368048 02147432 02238318 02237601 02267993 02171805 02231733 02368668 02039540 01912054 Apo-Atenol 50mg tab CO Atenolol 50mg tab Jamp-Atenolol 50mg tab Mar-Atenolol 50mg tab MINT-Atenol 50mg tab MYLAN-Atenolol 50mg tab phl-Atenolol 50mg tab pms-Atenolol 50mg tab RAN-Atenol 50mg tab ratio-Atenolol 50mg tab Sandoz Atenolol 50mg tab Septa-Atenolol 50mg tab Tenormin 50mg tab Apo-Atenol 100mg tab CO Atenolol 100mg tab Jamp-Atenolol 100mg tab Mar-Atenolol 100mg tab MINT-Atenol 100mg tab MYLAN-Atenolol 100mg tab phl-Atenolol 100mg tab pms-Atenolol 100mg tab RAN-Atenolol 100mg tab ratio-Atenolol 100mg tab Sandoz Atenolol 100mg tab Septa-Atenolol 100mg tab Tenormin 100mg tab Teva-Atenolol 100mg tab APX COB JPC MAR MNT MYL PHL PMS RAN TEV SDZ SPT AZE APX COB JPC MAR MNT MYL PHL PMS RAN TEV SDZ SPT AZE TEV 0.2069 0.2069 0.2069 0.2069 0.2069 0.2069 0.2069 0.2069 0.2069 0.2069 0.2069 0.2069 0.2069 0.3401 0.3401 0.3401 0.3401 0.3401 0.3401 0.3401 0.3401 0.3401 0.3401 0.3401 0.3401 0.3401 0.3401 02248763 02049961 02302918 Apo-Atenidone 50/25mg tab Tenoretic 50/25mg tab Teva-Atenolol/Chlorthalidone 50/25mg tab APX AZE TEV 0.3195 0.3195 0.3195 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 6 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength atenolol 100mg & chlorthalidone 25mg tab atorvastatin 10mg tab atorvastatin 20mg tab atorvastatin 40mg tab atorvastatin 80mg tab DIN 02248764 Brand Apo-Atenidone 100/25mg tab MFR MRP APX 0.5236 02049988 02302926 02295261 02348705 02310899 02288346 02230711 02373203 02302675 02313448 02313707 02350297 02324946 Tenoretic 100/25mg tab Teva-Atenolol/Chlorthalidone 100/25mg tab Apo-Atorvastatin 10mg tab Atorvastatin 10mg tab (SAS) CO Atorvastatin 10mg tab GD-Atorvastatin 10mg tab Lipitor 10mg tab MYLAN-Atorvastatin 10mg tab Novo-Atorvastatin 10mg tab pms-Atorvastatin 10mg tab RAN-Atorvastatin 10mg tab ratio-Atorvastatin 10mg tab Sandoz Atorvastatin 10mg tab AZE TEV APX SAS COB GMD PFI MYL TEV PMS RAN TEV SDZ 0.5236 0.5236 0.3138 0.3138 0.3138 0.3138 0.3138 0.3138 0.3138 0.3138 0.3138 0.3138 0.3138 02295288 02348713 02310902 02288354 02230713 02373211 02302683 02313456 02313715 02350319 02324954 02295296 02348721 02310910 02288362 02230714 02373238 02302691 02313464 02313723 02350327 02324962 02295318 02348748 02310929 02288370 02243097 02373246 02302713 02313472 Apo-Atorvastatin 20mg tab Atorvastatin 20mg tab (SAS) CO Atorvastatin 20mg tab GD-Atorvastatin 20mg tab Lipitor 20mg tab MYLAN-Atorvastatin 20mg tab Novo-Atorvastatin 20mg tab pms-Atorvastatin 20mg tab RAN-Atorvastatin 20mg tab ratio-Atorvastatin 20mg tab Sandoz Atorvastatin 20mg tab Apo-Atorvastatin 40mg tab Atorvastatin 40mg tab (SAS) CO Atorvastatin 40mg tab GD-Atorvastatin 40mg tab Lipitor 40mg tab MYLAN-Atorvastatin 40mg tab Novo-Atorvastatin 40mg tab pms-Atorvastatin 40mg tab RAN-Atorvastatin 40mg tab ratio-Atorvastatin 40mg tab Sandoz Atorvastatin 40mg tab Apo-Atorvastatin 80mg tab Atorvastatin 80mg tab (SAS) CO Atorvastatin 80mg tab GD-Atorvastatin 80mg tab Lipitor 80mg tab MYLAN-Atorvastatin 80mg tab Novo-Atorvastatin 80mg tab pms-Atorvastatin 80mg tab APX SAS COB GMD PFI MYL TEV PMS RAN TEV SDZ APX SAS COB GMD PFI MYL TEV PMS RAN TEV SDZ APX SAS COB GMD PFI MYL TEV PMS 0.3922 0.3922 0.3922 0.3922 0.3922 0.3922 0.3922 0.3922 0.3922 0.3922 0.3922 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 0.4216 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 7 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength atorvastatin 80mg tab DIN 02313758 02350335 02324970 Brand RAN-Atorvastatin 80mg tab ratio-Atorvastatin 80mg tab Sandoz Atorvastatin 80mg tab MFR MRP RAN 0.4216 TEV 0.4216 SDZ 0.4216 azathioprine 50mg tab 02242907 02343002 00004596 02231491 02236819 Apo-Azathioprine 50mg tab Azathioprine 50mg tab Imuran 50mg tab MYLAN-Azathioprine 50mg tab Teva-Azathioprine 50mg tab APX SAS GSK MYL TEV 0.3366 0.3366 0.3366 0.3366 0.3366 azithromycin 250mg tab (exception status) 02247423 Apo-Azithromycin 250mg tab APX 1.7290 azithromycin 600mg tab (exception status) 02330881 02255340 02274531 02278359 02267845 02278588 02261634 02275287 02265826 02212021 02330911 Azithromycin 250mg tab CO Azithromycin 250mg tab GD-Azithromycin 250mg tab MYLAN-Azithromycin 250mg tab Novo-Azithromycin 250mg tab phl-Azithromycin 250mg tab pms-Azithromycin 250mg tab ratio-Azithromycin 250mg tab Sandoz Azithromycin 250mg tab Zithromax 250mg tab Azithromycin 600mg tab SAS COB GMD MYL TEV PHL PMS TEV SDZ PFI SAS 1.7290 1.7290 1.7290 1.7290 1.7290 1.7290 1.7290 1.7290 1.7290 1.7290 6.0000 02256088 02261642 02231143 02315157 CO Azithromycin 600mg tab pms-Azithromycin 600mg tab Zithromax 600mg tab Novo-Azithromycin Pediatric 100mg/5mL susp COB PMS PFI TEV 6.0000 6.0000 6.0000 0.3956 02274388 02332388 02223716 02315165 pms-Azithromycin POS 100mg/5mL susp Sandoz Azithromycin POS 100mg/5mL susp Zithromax POS 100mg/5mL susp Novo-Azithromycin Pediatric 200mg/5mL susp PMS SDZ PFI TEV 0.3956 0.3956 0.3956 0.5604 02274396 02332396 02223724 02139332 02287021 00455881 02088398 02236963 02063735 02236507 02139391 02287048 00636576 02088401 pms-Azithromycin POS 200mg/5mL susp Sandoz Azithromycin POS 200mg/5mL susp Zithromax POS 200mg/5mL susp Apo-Baclofen 10mg tab Baclofen 10mg tab Lioresal 10mg tab MYLAN-Baclofen 10mg tab phl-Baclofen 10mg tab pms-Baclofen 10mg tab ratio-Baclofen 10mg tab Apo-Baclofen 20mg tab Baclofen 20mg tab Lioresal DS 20mg tab MYLAN-Baclofen 20mg tab PMS SDZ PFI APX SAS NVR MYL PHL PMS TEV APX SAS NVR MYL 0.5604 0.5604 0.5604 0.2403 0.2403 0.2403 0.2403 0.2403 0.2403 0.2403 0.4676 0.4676 0.4676 0.4676 azithromycin pos 100mg/5mL susp (exception status) azithromycin pos 200mg/5mL susp (exception status) baclofen 10mg tab baclofen 20mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 8 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength baclofen 20mg tab DIN 02236964 02063743 02236508 Brand phl-Baclofen 20mg tab pms-Baclofen 20mg tab ratio-Baclofen 20mg tab MFR MRP PHL 0.4676 PMS 0.4676 TEV 0.4676 beclomethasone dipropionate 50mcg/dose aqueous nasal spray 02238796 Apo-Beclo 50mcg/dose aq nasal sp APX 0.0613 02172712 MYLAN-Beclo 50mcg/dose aq nasal sp MYL 0.0613 benazepril 5mg tab 02290332 00885835 Benazepril 5mg tab Lotensin 5mg tab AAP NVR 0.6051 0.6051 benazepril 10mg tab 02290340 00885843 02273918 00885851 Benazepril 10mg tab Lotensin 10mg tab (discontinued) Benazepril 20mg tab Lotensin 20mg tab AAP NVR AAP NVR 0.7156 0.7156 0.8210 0.8210 00426857 00587265 02239044 Benztropine 2mg tab PMS pms-Benztropine 2mg tab (discontinued) PMS Apo-Benzydamine 0.15% oral rinse (discontinued) APX 0.0503 0.0503 0.0290 02310422 02229777 Novo-Benzydamine 0.15% oral rinse pms-Benzydamine 0.15% oral rinse TEV PMS 0.0290 0.0290 02374757 02280191 02243878 02374765 02280205 02247998 02237835 00028096 00716618 02357860 CO Betahistine 16mg tab Novo-Betahistine 16mg tab Serc 16mg tab CO Betahistine 24mg tab Novo-Betahistine 24mg tab Serc 24mg tab Betaject 6mg/mL inj Celestone soluspan 6mg/mL inj Betaderm 0.05% cr Celestoderm-V/2 0.05% cr COB TEV SPH COB TEV SPH SDZ SCH TAR VAL 0.1770 0.1770 0.1770 0.3933 0.3933 0.3933 9.5300 9.5300 0.0596 0.0596 betamethasone 17 valerate 0.1% cr 00716626 02357844 Betaderm 0.1% cr Celestoderm-V 0.1% cr TAR VAL 0.0889 0.0889 betamethasone dipropionate 0.05% cr 00323071 01925350 Diprosone 0.05% cr Taro-Sone 0.05% cr SCH TAR 0.2048 0.2048 betamethasone dipropionate 0.05% glycol cr 00688622 Diprolene 0.05% glycol cr SCH 0.5187 00849650 00862975 ratio-Topilene 0.05% glycol cr Diprolene 0.05% glycol lot TEV SCH 0.5187 0.5620 01927914 ratio-Topilene 0.05% glycol lot TEV 0.5620 00629367 Diprolene 0.05% glycol oint SCH 0.5187 benazepril 20mg tab benztropine mesylate 2mg tab benzydamine 0.15% oral rinse (exception status) betahistine 16mg tab (exception status) betahistine 24mg tab (exception status) betamethasone 6mg/mL inj betamethasone 17 valerate 0.05% cr betamethasone dipropionate 0.05% glycol lot betamethasone dipropionate 0.05% glycol oint 00849669 ratio-Topilene 0.05% glycol oint TEV 0.5187 betamethasone dipropionate 0.05% lot 00417246 00809187 Diprosone 0.05% lot ratio-Topisone 0.05% lot SCH TEV 0.1980 0.1980 betamethasone dipropionate 0.05% oint 00344923 00805009 Diprosone 0.05% oint ratio-Topisone 0.05% oint SCH TEV 0.2152 0.2152 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 9 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength bethamethasone dipropionate 0.05% & salicylic acid 2% lot bicalutamide 50mg tab bisoprolol 5mg tab bisoprolol 10mg tab boceprevir 200mg cap (exception status) bosentan 62.5mg tab (exception status) bosentan 125mg tab (exception status) brimonidine 0.2% oph sol bromazepam 1.5mg tab bromazepam 3mg tab DIN 00578428 Brand Diprosalic 0.05%/2% lot MFR MRP SCH 0.4228 02245688 ratio-Topisalic 0.05%/2% lot TEV 0.4228 02296063 02325985 02184478 02274337 02357216 02302403 02270226 02275589 02371324 02277700 02276089 02256134 02391589 02384418 02267470 02302632 02247439 02256177 02391597 02384426 02267489 02302640 02247440 Apo-Bicalutamide 50mg tab Bicalutamide 50mg tab Casodex 50mg tab CO Bicalutamide 50mg tab Jamp-Bicalutamide 50mg tab MYLAN-Bicalutamide 50mg tab Novo-Bicalutamide 50mg tab pms-Bicalutamide 50mg tab RAN-Bicalutamide 50mg Tab ratio-Bicalutamide 50mg tab Sandoz Bicalutamide 50mg tab Apo-Bisoprolol 5mg tab Bisoprolol 5mg tab MYLAN-Bisoprolol 5mg tab Novo-Bisoprolol 5mg tab pms-Bisoprolol 5mg tab Sandoz Bisoprolol 5mg tab Apo-Bisoprolol 10mg tab Bisoprolol 10mg tab MYLAN-Bisoprolol 10mg tab Novo-Bisoprolol 10mg tab pms-Bisoprolol 10mg tab Sandoz Bisoprolol 10mg tab APX AHC AZE COB JPC MYL TEV PMS RAN TEV SDZ APX SAS MYL TEV PMS SDZ APX SAS MYL TEV PMS SDZ 2.3188 2.3188 2.3188 2.3188 2.3188 2.3188 2.3188 2.3188 2.3188 2.3188 2.3188 0.1391 0.1391 0.1391 0.1391 0.1391 0.1391 0.2030 0.2030 0.2030 0.2030 0.2030 0.2030 02370816 02386194 02383497 02383012 02386275 02244981 Victrelis 200mg cap CO Bosentan 62.5mg tab MYLAN-Bosentan 62.5mg tab pms-Bosentan 62.5mg tab Sandoz Bosentan 62.5mg tab Tracleer 62.5mg tab FRS COB MYL PMS SDZ ACT 22.4625 22.4625 22.4625 22.4625 22.4625 02386208 02383500 02383020 02386283 02244982 02236876 02260077 02246284 02243026 02305429 CO Bosentan 125mg tab MYLAN-Bosentan 125mg tab pms-Bosentan 125mg tab Sandoz Bosentan 125mg tab Tracleer 125mg tab Alphagan 0.2% oph sol Apo-Brimonidine 0.2% oph sol pms-Brimonidine 0.2% oph sol ratio-Brimonidine 0.2% oph sol Sandoz Brimonidine 0.2% oph sol COB MYL PMS SDZ ACT ALL APX PMS TEV SDZ 22.4625 22.4625 22.4625 22.4625 22.4625 1.1550 1.1550 1.1550 1.1550 1.1550 02177153 02177161 00518123 Apo-Bromazepam 1.5mg tab Apo-Bromazepam 3mg tab Lectopam 3mg tab APX APX HLR 0.0693 0.0525 0.0525 13.5625 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 10 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength bromazepam 3mg tab DIN 02230584 Brand Novo-Bromazepam 3mg tab MFR MRP TEV 0.0525 bromazepam 6mg tab 02177188 00518131 02230585 Apo-Bromazepam 6mg tab Lectopam 6mg tab Novo-Bromazepam 6mg tab APX HLR TEV 0.0767 0.0767 0.0767 bromocriptine mesylate 2.5mg tab 02087324 02230454 Apo-Bromocriptine 2.5mg tab Apo-Bromocriptine 5mg cap APX APX 0.9782 1.4644 02241003 MYLAN-Budesonide 64mcg/mL aq nasal spray MYL 0.0843 02231923 Rhinocort 64mcg/mL aq nasal spray AZE 0.0843 02391562 02325373 02285657 02275074 02391570 02313421 02285665 02275082 02237825 Bupropion 100mg SR tab pms-Bupropion 100mg SR tab ratio-Bupropion 100mg SR tab Sandoz Bupropion 100mg SR tab Bupropion 150mg SR tab pms-Bupropion 150mg SR tab ratio-Bupropion 150mg SR tab Sandoz Bupropion 150mg SR tab Wellbutrin 150mg SR tab SAS PMS TEV SDZ SAS PMS TEV SDZ BVL 0.2167 0.2167 0.2167 0.2167 0.3236 0.3236 0.3236 0.3236 0.3236 02211076 02231492 02230942 02242504 Apo-Buspirone 10mg tab Novo-Buspirone 10mg tab pms-Buspirone 10mg tab Apo-Butorphanol nasal sp APX TEV PMS APX 0.3798 0.3798 0.3798 3.7683 02301407 02242471 02247585 CO Cabergoline 0.5mg tab Dostinex 0.5mg tab Apo-Calcitonin 200iu/dose nasal spray COB 10.5238 SQI 10.5238 APX 1.7254 02240775 02261766 02365359 02239091 02388928 02379279 02376539 02386518 02379139 02391198 02326965 02366312 02365367 02239092 02388936 02379287 02376547 02386526 Miacalcin 200iu/dose nasal spray Sandoz Calcitonin NS 200iu/dose nasal spray Apo-Candesartan 8mg tab Atacand 8mg tab Candesartan 8mg tab Candesartan Cilexetil 8mg tab CO Candesartan 8mg tab Jamp-Candesartan 8mg tab MYLAN-Candesartan 8mg tab pms-Candesartan 8mg tab Sandoz Candesartan 8mg tab Teva-Candesartan 8mg tab Apo-Candesartan 16mg tab Atacand 16mg tab Candesartan 16mg tab Candesartan Cilexetil 16mg tab CO Candesartan 16mg tab Jamp-Candesartan 16mg tab NVR SDZ APX AZE SAS AHI COB JPC MYL PMS SDZ TEV APX AZE SAS AHI COB JPC bromocriptine mesylate 5mg cap budesonide 64mcg/dose aqueous nasal spray bupropion 100mg SR tab bupropion 150mg SR tab buspirone HCl 10mg tab butorphanol 10mg/mL nasal sp (exception status) cabergoline 0.5mg tab (exception status) calcitonin 200iu/dose nasal spray (exception status) candesartan 8mg tab candesartan 16mg tab 1.7254 1.7254 0.4100 0.4100 0.4100 0.4100 0.4100 0.4100 0.4100 0.4100 0.4100 0.4100 0.4100 0.4100 0.4100 0.4100 0.4100 0.4100 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 11 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength candesartan 16mg tab DIN 02379147 02391201 02326973 02366320 Brand MYLAN-Candesartan 16mg tab pms-Candesartan 16mg tab Sandoz Candesartan 16mg tab Teva-Candesartan 16mg tab MFR MRP MYL 0.4100 PMS 0.4100 SDZ 0.4100 TEV 0.4100 candesartan 32mg tab 02311658 02379295 02376555 02386534 02379155 02391228 02392267 02366339 02367866 Atacand 32mg tab Candesartan Cilexetil 32mg tab CO Candesartan 32mg tab Jamp-Candesartan 32mg tab MYLAN-Candesartan 32mg tab pms-Candesartan 32mg tab Sandoz Candesartan 32mg tab Teva-Candesartan 32mg tab Apo-Candesartan/HCTZ 16/12.5mg tab AZE AHI COB JPC MYL PMS SDZ TEV APX 0.4193 0.4193 0.4193 0.4193 0.4193 0.4193 0.4193 0.4193 0.4193 02244021 02394804 02388650 02374897 02391295 02327902 00893595 02163551 01942964 Atacand Plus 16/12.5mg tab Candesartan/HCTZ 16/12.5mg tab CO Candesartan/HCT 16/12.5mg tab MYLAN-Candesartan HCTZ 16/12.5mg tab pms-Candesartan HCTZ 16/12.5mg tab Sandoz Candesartan Plus 16/12.5mg tab Apo-Capto 12.5mg tab MYLAN-Captopril 12.5mg tab Novo-Captoril 12.5mg tab AZE SAS COB MYL PMS SDZ APX MYL TEV 0.4193 0.4193 0.4193 0.4193 0.4193 0.4193 0.1060 0.1060 0.1060 captopril 25mg tab 00893609 02163578 01942972 Apo-Capto 25mg tab MYLAN-Captopril 25mg tab Novo-Captoril 25mg tab APX MYL TEV 0.1500 0.1500 0.1500 captopril 50mg tab 00893617 00546291 02163586 01942980 00893625 02163594 01942999 00402699 00010405 00782718 02231542 02261855 02244403 00369810 02231540 02261863 02244404 00665088 02241882 Apo-Capto 50mg tab Capoten 50mg tab (discontinued) MYLAN-Captopril 50mg tab Novo-Captoril 50mg tab Apo-Capto 100mg tab MYLAN-Captopril 100mg tab Novo-Captoril 100mg tab Apo-Carbamazepine 200mg tab (discontinued) Tegretol 200mg tab Teva-Carbamazepine 200mg tab pms-Carbamazepine 100mg chewable tab Sandoz Carbamazepine 100mg chewable tab Taro-Carbamazepine 100mg chewable tab Tegretol 100mg chewable tab pms-Carbamazepine 200mg chewable tab Sandoz Carbamazepine 200mg chewable tab Taro-Carbamazepine 200mg chewable tab Tegretol 200mg chewable tab MYLAN-Carbamazepine 200mg CR tab APX BRI MYL TEV APX MYL TEV APX NVR TEV PMS SDZ TAR NVR PMS SDZ TAR NVR MYL 0.2795 0.2795 0.2795 0.2795 0.5198 0.5198 0.5198 0.0795 0.0795 0.0795 0.0572 0.0572 0.0572 0.0572 0.1128 0.1128 0.1128 0.1128 0.1401 candesartan 16mg & hydrochlorothiazide 12.5mg tab captopril 12.5mg tab captopril 100mg tab carbamazepine 200mg tab carbamazepine 100mg chewable tab carbamazepine 200mg chewable tab carbamazepine 200mg cr tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 12 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength carbamazepine 200mg cr tab DIN 02231543 02261839 00773611 Brand pms-Carbamazepine 200mg CR tab Sandoz Carbamazepine 200mg CR tab Tegretol 200mg CR tab MFR MRP PMS 0.1401 SDZ 0.1401 NVR 0.1401 carbamazepine 400mg cr tab 02241883 02231544 02261847 00755583 02247933 02364913 02368897 02347512 02248752 02245914 02268027 02252309 02338068 MYLAN-Carbamazepine 400mg CR tab pms-Carbamazepine 400mg CR tab Sandoz Carbamazepine 400mg CR tab Tegretol 400mg CR tab Apo-Carvedilol 3.125mg tab Carvedilol 3.125mg tab Jamp-Carvedilol 3.125mg tab MYLAN-Carvedilol 3.125mg tab phl-Carvedilol 3.125mg tab pms-Carvedilol 3.125mg tab RAN-Carvedilol 3.125mg tab ratio-Carvedilol 3.125mg tab Zym-Carvedilol 3.125mg tab MYL PMS SDZ NVR APX SAS JPC MYL PHL PMS RAN TEV ZYM 0.2801 0.2801 0.2801 0.2801 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 carvedilol 6.25mg tab (exception status) 02247934 02364921 02368900 02347520 02248753 02245915 02268035 02252317 02338092 Apo-Carvedilol 6.25mg tab Carvedilol 6.25mg tab Jamp-Carvedilol 6.25mg tab MYLAN-Carvedilol 6.25mg tab phl-Carvedilol 6.25mg tab pms-Carvedilol 6.25mg tab RAN-Carvedilol 6.25mg tab ratio-Carvedilol 6.25mg tab Zym-Carvedilol 6.25mg tab APX SAS JPC MYL PHL PMS RAN TEV ZYM 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 carvedilol 12.5mg tab (exception status) 02247935 02364948 02368919 02347555 02248754 02245916 02268043 02252325 02338106 02247936 02364956 02368927 02347571 02248755 02245917 02268051 02252333 02338114 Apo-Carvedilol 12.5mg tab Carvedilol 12.5mg tab Jamp-Carvedilol 12.5mg tab MYLAN-Carvedilol 12.5mg tab phl-Carvedilol 12.5mg tab pms-Carvedilol 12.5mg tab RAN-Carvedilol 12.5mg tab ratio-Carvedilol 12.5mg tab Zym-Carvedilol 12.5mg tab Apo-Carvedilol 25mg tab Carvedilol 25mg tab Jamp-Carvedilol 25mg tab MYLAN-Carvedilol 25mg tab phl-Carvedilol 25mg tab pms-Carvedilol 25mg tab RAN-Carvedilol 25mg tab ratio-Carvedilol 25mg tab Zym-Carvedilol 25mg tab APX SAS JPC MYL PHL PMS RAN TEV ZYM APX SAS JPC MYL PHL PMS RAN TEV ZYM 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 0.4728 02240774 02235134 Apo-Cefadroxil 500mg cap Novo-Cefadroxil 500mg cap APX TEV 0.8421 0.8421 carvedilol 3.125mg tab (exception status) carvedilol 25mg tab (exception status) cefadroxil 500mg cap Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 13 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength cefazolin sodium 500mg/vial inj DIN 02108119 02308932 Brand Cefazolin Sodium 500mg/vial inj Cefazolin Sodium 500mg/vial inj MFR MRP TEV 4.0000 SDZ 4.0000 cefazolin sodium 1g/vial inj 02108127 02297205 02308959 Cefazolin Sodium 1g/vial inj Cefazolin Sodium 1g/vial inj Cefazolin Sodium 1g/vial inj TEV APX SDZ 6.0000 6.0000 6.0000 cefprozil 125mg/5mL o/l 02293943 02347261 02163675 02329204 02303426 Apo-Cefprozil 125mg/5mL o/l Auro-Cefprozil 125mg/5mL Susp Cefzil 125mg/5mL o/l RAN-Cefprozil 125mg/5mL o/l Sandoz Cefprozil 125mg/5mL o/l APX ARO BRI RAN SDZ 0.0593 0.0593 0.0593 0.0593 0.0593 cefprozil 250mg/5mL o/l 02293951 02347288 02163683 02293579 02303434 02292998 02347245 02163659 02293528 02302179 02293005 02347253 02163667 02293536 02302187 Apo-Cefprozil 250mg/5mL o/l Auro-Cefprozil 250mg/5mL Susp Cefzil 250mg/5mL o/l RAN-Cefprozil 250mg/5mL o/l Sandoz Cefprozil 250mg/5mL o/l Apo-Cefprozil 250mg tab Auro-Cefprozil 250mg tab Cefzil 250mg tab RAN-Cefprozil 250mg tab Sandoz Cefprozil 250mg tab Apo-Cefprozil 500mg tab Auro-Cefprozil 500mg tab Cefzil 500mg tab RAN-Cefprozil 500mg tab Sandoz Cefprozil 500mg tab APX ARO BRI RAN SDZ APX ARO BRI RAN SDZ APX ARO BRI RAN SDZ 0.1185 0.1185 0.1185 0.1185 0.1185 0.6064 0.6064 0.6064 0.6064 0.6064 1.1891 1.1891 1.1891 1.1891 1.1891 02292866 00657387 02292874 02292270 00657417 Ceftriaxone 0.25g/vial inj Rocephin 0.25g/vial inj (discontinued) Ceftriaxone 1g/vial inj (APX) Ceftriaxone 1g/vial inj (SDZ) Rocephin 1g/vial inj (discontinued) APX HLR APX SDZ HLR 7.5250 7.5250 12.4950 12.4950 12.4950 02292882 02292289 02244393 02344823 02212277 02242656 02244394 02344831 02212285 02242657 Ceftriaxone 2g/vial inj (APX) Ceftriaxone 2g/vial inj (SDZ) Apo-Cefuroxime 250mg tab Auro-Cefuroxime 250mg tab Ceftin 250mg tab ratio-Cefuroxime 250mg tab Apo-Cefuroxime 500mg tab Auro-Cefuroxime 500mg tab Ceftin 500mg tab ratio-Cefuroxime 500mg tab APX 24.1400 SDZ 24.1400 APX 0.7237 ARO 0.7237 GSK 0.7237 TEV 0.7237 APX 1.4337 ARO 1.4337 GSK 1.4337 TEV 1.4337 02239941 02239942 00342106 00342092 Celebrex 100mg cap Celebrex 200mg cap Novo-Lexin 125mg/5mL susp Novo-Lexin 250mg/5mL susp PFI PFI TEV TEV cefprozil 250mg tab cefprozil 500mg tab ceftriaxone 0.25g/vial inj ceftriaxone 1g/vial inj ceftriaxone 2g/vial inj cefuroxime axetil 250mg tab cefuroxime axetil 500mg tab celecoxib 100mg cap celecoxib 200mg cap cephalexin monohydrate 25mg o/l cephalexin monohydrate 50mg o/l 0.2625 0.5250 0.0860 0.1351 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 14 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength cephalexin monohydrate 250mg tab DIN 00768723 00583413 Brand Apo-Cephalex 250mg tab Novo-Lexin 250mg tab MFR MRP APX 0.2250 TEV 0.2250 cephalexin monohydrate 500mg tab 00768715 00583421 Apo-Cephalex 500mg tab Novo-Lexin 500mg tab APX TEV 0.4500 0.4500 cetirizine 10mg tab (exception status) 02231603 02231603 02315955 02223554 APC-Cetirizine 10mg tab Apo-Cetirizine 10mg tab Extra Strength Allergy Relief 10mg tab Reactine 10mg tab APX APX PDP JNJ 0.4083 0.4083 0.4083 0.4083 chlordiazepoxide HCl 5mg & clidinium Br 2.5mg cap 00618454 Apo-Chlorax 5mg/2.5mg cap APX 0.2231 00115630 Librax 5mg/2.5mg cap VLN 0.2231 chloroquine phosphate 250mg tab chlorpromazine 25mg/mL inj cilazapril 1mg tab 00021261 Novo-Chloroquine 250mg tab TEV 0.4020 00743518 02291134 02350963 02283778 02266350 02280442 Chlorpromazine 25mg/mL inj Apo-Cilazapril 1mg tab Cilazapril 1mg tab MYLAN-Cilazapril 1mg tab Novo-Cilazapril 1mg tab pms-Cilazapril 1mg tab SDZ APX SAS MYL TEV PMS 1.1100 0.2492 0.2492 0.2492 0.2492 0.2492 cilazapril 2.5mg tab 02291142 02350971 01911473 02283786 02266369 02280450 02291150 02350998 01911481 02283794 02266377 02280469 02284987 Apo-Cilazapril 2.5mg tab Cilazapril 2.5mg tab Inhibace 2.5mg tab MYLAN-Cilazapril 2.5mg tab Novo-Cilazapril 2.5mg tab pms-Cilazapril 2.5mg tab Apo-Cilazapril 5mg tab Cilazapril 5mg tab Inhibace 5mg tab MYLAN-Cilazapril 5mg tab Novo-Cilazapril 5mg tab pms-Cilazapril 5mg tab Apo-Cilazapril/HCTZ 5mg/12.5mg tab APX SAS HLR MYL TEV PMS APX SAS HLR MYL TEV PMS APX 0.2513 0.2513 0.2513 0.2513 0.2513 0.2513 0.2919 0.2919 0.2919 0.2919 0.2919 0.2919 0.4170 02181479 02313731 00584215 00487872 02227444 00600059 02227452 Inhibace Plus 5mg/12.5mg tab Novo-Cilazapril/HCTZ 5mg/12.5mg tab Apo-Cimetidine 200mg tab Apo-Cimetidine 300mg tab MYLAN-Cimetidine 300mg tab Apo-Cimetidine 400mg tab MYLAN-Cimetidine 400mg tab HLR TEV APX APX MYL APX MYL 0.4170 0.4170 0.0860 0.0860 0.0860 0.1350 0.1350 00600067 02227460 00749494 Apo-Cimetidine 600mg tab MYLAN-Cimetidine 600mg tab Apo-Cimetidine 800mg tab APX MYL APX 0.1702 0.1702 0.2530 01945270 Ciloxan 0.3% oph sol ALC 0.7920 02253933 pms-Ciprofloxacin 0.3% oph sol PMS 0.7920 cilazapril 5mg tab cilazapril 5mg & hydrochlorothiazide 12.5mg tab cimetidine 200mg tab cimetidine 300mg tab cimetidine 400mg tab cimetidine 600mg tab cimetidine 800mg tab ciprofloxacin 0.3% oph sol (exception status) Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 15 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength ciprofloxacin 0.3% oph sol (exception status) ciprofloxacin 250mg tab (exception status) DIN 02387131 Brand Sandoz Ciprofloxacin 0.3% oph sol MFR MRP SDZ 0.7920 02229521 02381907 02155958 02353318 02247339 02380358 02379686 02317427 02245647 02161737 02248437 02303728 02246825 02248756 02379627 Apo-Ciproflox 250mg tab Auro-Ciprofloxacin 250mg tab Cipro 250mg tab Ciprofloxacin 250mg tab CO Ciprofloxacin 250mg tab Jamp-Ciprofloxacin 250mg tab Mar-Ciprofloxacin 250mg tab MINT-Ciprofloxacin 250mg tab MYLAN-Ciprofloxacin 250mg tab Novo-Ciprofloxacin 250mg tab pms-Ciprofloxacin 250mg tab RAN-Ciproflox 250mg tab ratio-Ciprofloxacin 250mg tab Sandoz Ciprofloxacin 250mg tab Septa-Ciprofloxacin 250mg tab APX ARO BAY SAS COB JPC MAR MNT MYL TEV PMS RAN TEV SDZ SPT 0.8660 0.8660 0.8660 0.8660 0.8660 0.8660 0.8660 0.8660 0.8660 0.8660 0.8660 0.8660 0.8660 0.8660 0.8660 ciprofloxacin 500mg tab (exception status) 02229522 02381923 02155966 02353326 02247340 02380366 02379694 02317435 02245648 02161745 02248438 02303736 02246826 02248757 02379635 Apo-Ciproflox 500mg tab Auro-Ciprofloxacin 500mg tab Cipro 500mg tab Ciprofloxacin 500mg tab CO Ciprofloxacin 500mg tab Jamp-Ciprofloxacin 500mg tab Mar-Ciprofloxacin 500mg tab MINT-Ciprofloxacin 500mg tab MYLAN-Ciprofloxacin 500mg tab Novo-Ciprofloxacin 500mg tab pms-Ciprofloxacin 500mg tab RAN-Ciproflox 500mg tab ratio-Ciprofloxacin 500mg tab Sandoz Ciprofloxacin 500mg tab Septa-Ciprofloxacin 500mg tab APX ARO BAY SAS COB JPC MAR MNT MYL TEV PMS RAN TEV SDZ SPT 0.9770 0.9770 0.9770 0.9770 0.9770 0.9770 0.9770 0.9770 0.9770 0.9770 0.9770 0.9770 0.9770 0.9770 0.9770 ciprofloxacin 750mg tab (exception status) 02229523 02381931 02155974 02353334 02247341 02380374 02379708 02317443 02245649 02161753 02248439 02303744 02246827 Apo-Ciproflox 750mg tab Auro-Ciprofloxacin 750mg tab Cipro 750mg tab Ciprofloxacin 750mg tab CO Ciprofloxacin 750mg tab Jamp-Ciprofloxacin 750mg tab Mar-Ciprofloxacin 750mg tab MINT-Ciprofloxacin 750mg tab MYLAN-Ciprofloxacin 750mg tab Novo-Ciprofloxacin 750mg tab pms-Ciprofloxacin 750mg tab RAN-Ciproflox 750mg tab ratio-Ciprofloxacin 750mg tab APX ARO BAY SAS COB JPC MAR MNT MYL TEV PMS RAN TEV 1.7891 1.7891 1.7891 1.7891 1.7891 1.7891 1.7891 1.7891 1.7891 1.7891 1.7891 1.7891 1.7891 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 16 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength ciprofloxacin 750mg tab (exception status) DIN 02248758 Brand Sandoz Ciprofloxacin 750mg tab MFR MRP SDZ 1.7891 02379643 Septa-Ciprofloxacin 750mg tab SPT 1.7891 citialopram 10mg tab 02273543 02270609 phl-Citalopram 10mg tab pms-Citalopram 10mg tab PHL PMS 0.4464 0.4464 citalopram 20mg tab 02246056 02275562 02239607 02353660 02306239 02248050 02313405 02371898 02304686 02246594 02293218 02248944 02248010 02285622 02252112 02248170 02355272 02246057 02275570 02239608 02353679 02306247 02248051 02313413 02371901 02304694 02246595 02293226 02248945 02248011 02285630 02252120 02248171 02355280 02274744 Apo-Citalopram 20mg tab Auro-Citalopram 20mg tab Celexa 20mg tab Citalopram 20mg tab Citalopram-Odan 20mg tab CO Citalopram 20mg tab Jamp-Citalopram 20mg tab Mar-Citalopram 20mg tab MINT-Citalopram 20mg tab MYLAN-Citalopram 20mg tab Novo-Citalopram 20mg tab phl-Citalopram 20mg tab pms-Citalopram 20mg tab RAN-Citalo 20mg tab ratio-Citalopram 20mg tab Sandoz Citalopram 20mg tab Septa-Citalopram 20mg tab Apo-Citalopram 40mg tab Auro-Citalopram 40mg tab Celexa 40mg tab Citalopram 40mg tab Citalopram-Odan 40mg tab CO Citalopram 40mg tab Jamp-Citalopram 40mg tab Mar-Citalopram 40mg tab MINT-Citalopram 40mg tab MYLAN-Citalopram 40mg tab Novo-Citalopram 40mg tab phl-Citalopram 40mg tab pms-Citalopram 40mg tab RAN-Citalo 40mg tab ratio-Citalopram 40mg tab Sandoz Citalopram 40mg tab Septa-Citalopram 40mg tab Apo-Clarithromycin 250mg tab APX ARO VLH SAS ODN COB JPC MAR MNT MYL TEV PHL PMS RAN TEV SDZ SPT APX ARO VLH SAS ODN COB JPC MAR MNT MYL TEV PHL PMS RAN TEV SDZ SPT APX 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.4661 0.5770 01984853 02248856 02247573 02361426 Biaxin BID 250mg tab MYLAN-Clarithromycin 250mg tab pms-Clarithromycin 250mg tab RAN-Clarithromycin 250mg tab ABB MYL PMS RAN 0.5770 0.5770 0.5770 0.5770 citalopram 40mg tab clarithromycin 250mg tab (exception status) Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 17 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength clarithromycin 250mg tab (exception status) DIN 02247818 Brand ratio-Clarithromycin 250mg tab MFR MRP TEV 0.5770 02266539 02248804 02274752 Sandoz Clarithromycin 250mg tab Teva-Clarithromycin 250mg tab Apo-Clarithromycin 500mg tab SDZ TEV APX 0.5770 0.5770 1.6293 02126710 02248857 02247574 02361434 02247819 02266547 02248805 Biaxin BID 500mg tab MYLAN-Clarithromycin 500mg tab pms-Clarithromycin 500mg tab RAN-Clarithromycin 500mg tab ratio-Clarithromycin 500mg tab Sandoz Clarithromycin 500mg tab Teva-Clarithromycin 500mg tab ABB MYL PMS RAN TEV SDZ TEV 1.6293 1.6293 1.6293 1.6293 1.6293 1.6293 1.6293 02390442 Accel-Clarithromycin 125mg/5mL o/l ACC 0.2047 02146908 02390450 Biaxin 125mg/5mL o/l Accel-Clarithromycin 250mg/5mL o/l ABB ACC 0.2047 0.3998 02244641 02230535 00260436 Biaxin 250mg/5mL o/l Clindamycin 150mg/mL (bulk) inj Dalacin C Phos 150mg/mL (bulk) inj ABB SDZ PFI 0.3998 3.3250 3.3250 clindamycin 150mg cap 02245232 00030570 02258331 02241709 Apo-Clindamycin 150mg cap Dalacin C 150mg cap MYLAN-Clindamycin 150mg cap Teva-Clindamycin 150mg cap APX PFI MYL TEV 0.3294 0.3294 0.3294 0.3294 clindamycin 300mg cap 02245233 02182866 02258358 02241710 Apo-Clindamycin 300mg cap Dalacin C 300mg cap MYLAN-Clindamycin 300mg cap Novo-Clindamycin 300mg cap APX PFI MYL TEV 0.6588 0.6588 0.6588 0.6588 clindamycin 150mg/mL inj 02230540 00260436 00582301 02266938 02244638 02221799 02238334 02244474 Clindamycin 150mg/mL inj Dalacin C Phos 150mg/mL inj Dalacin T 1% top sol Taro-Clindamycin 1% top sol Apo-Clobazam 10mg tab Frisium 10mg tab Novo-Clobazam 10mg tab pms-Clobazam 10mg tab SDZ PFI PFI TAR APX OVN TEV PMS 3.3250 3.3250 0.2260 0.2260 0.1538 0.1538 0.1538 0.1538 02213265 02024187 02093162 02309521 02232191 01910272 02245523 02213273 02026767 Dermovate 0.05% cr MYLAN-Clobetasol 0.05% cr Novo-Clobetasol 0.05% cr pms-Clobetasol 0.05% cr pms-Clobetasol 0.05% cr (discontinued) ratio-Clobetasol 0.05% cr Taro-Clobetasol 0.05% cr Dermovate 0.05% oint MYLAN-Clobetasol 0.05% oint TPH MYL TEV PMS PMS TEV TAR TPH MYL 0.2279 0.2279 0.2279 0.2279 0.2279 0.2279 0.2279 0.2279 0.2279 clarithromycin 500mg tab (exception status) clarithromycin 25mg/mL o/l (exception status) clarithromycin 50mg/mL o/l (exception status) clindamycin 150mg/mL (bulk) inj clindamycin 1% top sol clobazam 10mg tab clobetasol 17-propionate 0.05% cr clobetasol 17-propionate 0.05% oint Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 18 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength clobetasol 17-propionate 0.05% oint DIN 02126192 02309548 01910280 02245524 Brand Novo-Clobetasol 0.05% oint pms-Clobetasol 0.05% oint ratio-Clobetasol 0.05% oint Taro-Clobetasol 0.05% oint MFR MRP TEV 0.2279 PMS 0.2279 TEV 0.2279 TAR 0.2279 clobetasol 17-propionate 0.05% scalp lot 02213281 02216213 02232195 01910299 02245522 Dermovate 0.05% scalp lot MYLAN-Clobetasol 0.05% scalp lot pms-Clobetasol 0.05% scalp lot ratio-Clobetasol 0.05% scalp lot Taro-Clobetasol 0.05% scalp lot TPH MYL PMS TEV TAR 0.1990 0.1990 0.1990 0.1990 0.1990 clomipramine 10mg tab 00330566 02040786 02244816 Anafranil 10mg tab Apo-Clomipramine 10mg tab CO Clomipramine 10mg tab ORX APX COB 0.1290 0.1290 0.1290 clomipramine 25mg tab 00324019 02040778 02244817 00402591 02040751 02244818 02177889 02270641 02230950 02239024 02236948 02048701 02207818 00382825 02233960 02345676 Anafranil 25mg tab Apo-Clomipramine 25mg tab CO Clomipramine 25mg tab Anafranil 50mg tab Apo-Clomipramine 50mg tab CO Clomipramine 50mg tab Apo-Clonazepam 0.5mg tab CO Clonazepam 0.5mg tab MYLAN-Clonazepam 0.5mg tab Novo-Clonazepam 0.5mg tab phl-Clonazepam-R 0.5mg tab pms-Clonazepam 0.5mg tab pms-Clonazepam-R 0.5mg tab Rivotril 0.5mg tab Sandoz Clonazepam 0.5mg tab Zym-Clonazepam 0.5mg tab ORX APX COB ORX APX COB APX COB MYL TEV PHL PMS PMS HLR SDZ ZYM 0.1758 0.1758 0.1758 0.3237 0.3237 0.3237 0.0694 0.0694 0.0694 0.0694 0.0694 0.0694 0.0694 0.0694 0.0694 0.0694 clonazepam 1mg tab 02145235 02048728 phl-Clonazepam 1mg tab pms-Clonazepam 1mg tab PHL PMS 0.1487 0.1487 clonazepam 2mg tab 02177897 02270676 02230951 02239025 02145243 02048736 00382841 02233985 02303337 00519251 02304163 Apo-Clonazepam 2mg tab CO Clonazepam 2mg tab MYLAN-Clonazepam 2mg tab Novo-Clonazepam 2mg tab phl-Clonazepam 2mg tab pms-Clonazepam 2mg tab Rivotril 2mg tab Sandoz Clonazepam 2mg tab Zym-Clonazepam 2mg tab Dixarit 0.025mg tab Novo-Clonidine 0.025mg tab APX COB MYL TEV PHL PMS HLR SDZ ZYM BOE TEV 0.1196 0.1196 0.1196 0.1196 0.1196 0.1196 0.1196 0.1196 0.1196 0.1523 0.1523 00259527 02046121 00291889 Catapres 0.1mg tab Novo-Clonidine 0.1mg tab Catapres 0.2mg tab BOE TEV BOE 0.1358 0.1358 0.2424 clomipramine 50mg tab clonazepam 0.5mg tab clonidine HCl 0.025mg tab clonidine HCl 0.1mg tab clonidine HCl 0.2mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 19 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength clonidine HCl 0.2mg tab DIN 02046148 Brand Novo-Clonidine 0.2mg tab MFR MRP TEV 0.2424 clopidogrel 75mg tab (exception status) 02252767 02303027 02351536 02238682 02348004 02379813 02359316 02293161 Apo-Clopidogrel 75mg tab CO Clopidogrel 75mg tab MYLAN-Clopidogrel 75mg tab Plavix 75mg tab pms-Clopidogrel 75mg tab RAN-Clopidogrel 75mg tab Sandoz Clopidogrel 75mg tab Teva-Clopidogrel 75mg tab APX COB MYL BRI PMS RAN SDZ TEV 0.9206 0.9206 0.9206 0.9206 0.9206 0.9206 0.9206 0.9206 clorazepate dipotassium 3.75mg cap 00860689 Apo-Clorazepate 3.75mg cap APX 0.1476 clorazepate dipotassium 7.5mg cap 00860700 00860697 02150867 00812382 02150891 00812366 Apo-Clorazepate 7.5mg cap Apo-Clorazepate 15mg cap Canesten 1% cr Clotrimaderm 1% cr Canesten 1% vag cr Clotrimaderm 1% vag cr APX APX YNO TAR YNO TAR 0.1810 0.3259 0.0884 0.0884 0.1750 0.1750 02150905 00812374 00337765 00337773 Canesten 2% vag cr Clotrimaderm 2% vag cr Novo-Cloxin 250mg cap Novo-Cloxin 500mg cap YNO TAR TEV TEV 0.3500 0.3500 0.3515 0.6646 00337757 02046113 Novo-Cloxin 25mg/mL o/l pms-Sodium Cromoglycate 1% neb sol TEV PMS 0.0855 0.8351 01950541 02009277 02230621 02241500 02177145 02348853 02287064 02357127 02231353 02080052 02249359 02212048 02150689 02247073 02150662 02247074 02150670 Rhinaris-CS Anti-Allergic nasal mist Cromolyn 2% oph sol Opticrom 2% oph sol Vitamin B12 100mcg/mL inj Apo-Cyclobenzaprine 10mg tab Auro-Cyclobenzaprine 10mg tab Cyclobenzaprine 10mg tab Jamp-Cyclobenzaprine 10mg tab MYLAN-Cyclobenzaprine 10mg tab Novo-Cycloprine 10mg tab phl-Cyclobenzaprine 10mg tab pms-Cyclobenzaprine 10mg tab Neoral 25mg cap Sandoz Cyclosporine 25mg cap Neoral 50mg cap Sandoz Cyclosporine 50mg cap Neoral 100mg cap PMS PDP ALL SDZ APX ARO SAS JPC MYL TEV PHL PMS NVR SDZ NVR SDZ NVR 0.5292 0.9500 0.9500 1.4500 0.3727 0.3727 0.3727 0.3727 0.3727 0.3727 0.3727 0.3727 1.3050 1.3050 2.5450 2.5450 5.0900 02242821 02244324 Sandoz Cyclosporine 100mg cap Apo-Cyclosporine 100mg/mL o/l SDZ APX 5.0900 3.7708 02150697 Neoral 100mg/mL o/l NVR 3.7708 clorazepate dipotassium 15mg cap clotrimazole 1% cr clotrimazole 1% vag cr clotrimazole 2% vag cr cloxacillin 250mg cap cloxacillin 500mg cap cloxacillin 25mg/mL o/l cromoglycate sodium 1% unit dose inh sol cromoglycate sodium 2% nasal sol cromoglycate sodium 2% oph sol cyanocobalamin 100mcg/mL inj cyclobenzaprine HCl 10mg tab cyclosporine 25mg cap (exception status) cyclosporine 50mg cap (exception status) cyclosporine 100mg cap (exception status) cyclosporine 100mg/mL o/l (exception status) Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 20 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength cyproterone 50mg tab DIN 00704431 02245898 02390760 Brand Androcur 50mg tab Cyproterone 50mg tab MED-Cyproterone 50mg tab MFR MRP PMS 1.5283 AAP 1.5283 GMP 1.5283 dasatinib 50mg cap (exception status) 02293137 Sprycel 50mg cap BRI 78.0658 dasatinib 100mg cap (exception status) 02320193 02287420 Sprycel 100mg cap Exjade 125mg tab for susp BRI NVR 154.4823 11.0714 deferasirox 250mg tab (exception status) deferasirox 500mg tab (exception status) 02287439 Exjade 250mg tab for susp NVR 22.1421 02287447 Exjade 500mg tab for susp NVR 44.2850 deferoxamine 500mg/vial inj 01981242 02241600 02242055 Desferal 500mg/vial inj Desferrioxamine 500mg/vial inj pms-Deferoxamine 500mg/vial inj NVR HOS PMS 7.4800 7.4800 7.4800 desipramine 10mg tab desipramine 25mg tab desipramine 50mg tab 02216248 02216256 Apo-Desipramine 10mg tab Apo-Desipramine 25mg tab APX APX 0.3804 0.3804 02216264 Apo-Desipramine 50mg tab APX 0.6704 desipramine 75mg tab 02216272 02216280 Apo-Desipramine 75mg tab Apo-Desipramine 100mg tab APX APX 0.8915 0.8915 02284030 Apo-Desmopressin 0.1mg tab APX 0.4626 00824305 02287730 02304368 02284049 DDAVP 0.1mg tab Novo-Desmopressin 0.1mg tab pms-Desmopressin 0.1mg tab Apo-Desmopressin 0.2mg tab FEI TEV PMS APX 0.4626 0.4626 0.4626 0.9251 00824143 02287749 02304376 DDAVP 0.2mg tab Novo-Desmopressin 0.2mg tab pms-Desmopressin 0.2mg tab FEI TEV PMS 0.9251 0.9251 0.9251 02284995 DDAVP Melt 60mcg SL tab FEI 0.4626 02285002 DDAVP Melt 120mcg SL tab FEI 0.9251 02317192 Apri 21 tab 21 day APX 0.5436 02042487 Marvelon 21 tab 21 day ORG 0.5436 02317206 Apri 28 tab 28 day APX 0.4077 02042479 02229315 02229323 02261081 01964976 01964968 02250055 00489158 01964070 01977547 Marvelon 28 tab 28 day pms-Desonide 0.05% cr pms-Desonide 0.05% oint Apo-Dexamethasone 0.5mg tab pms-Dexamethasone 0.5mg tab pms-Dexamethasone 0.75mg tab Apo-Dexamethasone 4mg tab Dexasone 4mg tab pms-Dexamethasone 4mg tab Dexamethasone 4mg/mL inj ORG PMS PMS APX PMS PMS APX VLN PMS CYI 0.4077 0.3349 0.3177 0.1095 0.1095 0.4500 0.4265 0.4265 0.4265 1.6060 deferasirox 125mg tab (exception status) desipramine 100mg tab desmopressin 0.1mg tab (exception status) desmopressin 0.2mg tab (exception status) desmopressin 60mcg SL tab (exception status) desmopressin 120mcg SL tab (exception status) desogestrel 150mcg and ethinyl estradiol 30mcg tab (21) desogestrel 150mcg and ethinyl estradiol 30mcg tab (28) desonide 0.05% cr desonide 0.05% oint dexamethasone 0.5mg tab dexamethasone 0.75mg tab dexamethasone 4mg tab dexamethasone 4mg/mL inj Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 21 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength dexamethasone 4mg/mL inj DIN 00664227 Brand Dexamethasone 4mg/mL inj MFR MRP SDZ 1.6060 dexamethasone 0.1% oph/otic sol 00739839 Sandoz Dexamethasone 0.1% oph/otic sol SDZ 1.4060 diazepam 5mg tab 00362158 00013285 Apo-Diazepam 5mg tab Valium 5mg tab APX HLR 0.0650 0.0650 diazepam 10mg tab 00405337 00399728 00839175 00808539 02302616 02261952 Apo-Diazepam 10mg tab Diazepam 5mg/mL inj Apo-Diclo 25mg EC tab Novo-Difenac 25mg EC tab pms-Diclofenac 25mg EC tab Sandoz Diclofenac 25mg EC tab APX SDZ APX TEV PMS SDZ 0.0867 0.7850 0.1094 0.1094 0.1094 0.1094 diclofenac sodium 50mg EC tab 00839183 02352397 00808547 02302624 02261960 00514012 Apo-Diclo 50mg EC tab Diclofenac Sodium 50mg tab Novo-Difenac 50mg EC tab pms-Diclofenac 50mg EC tab Sandoz Diclofenac 50mg EC tab Voltaren 50mg EC tab APX SAS TEV PMS SDZ NVR 0.2333 0.2333 0.2333 0.2333 0.2333 0.2333 diclofenac sodium 75mg SR tab 02162814 02352400 02158582 02231504 02261901 00782459 02091194 02048698 02231505 02261944 00590827 Apo-Diclo 75mg SR tab Diclofenac Sodium 75mg SR tab Novo-Difenac 75mg SR tab pms-Diclofenac 75mg SR tab Sandoz Diclofenac 75mg SR tab Voltaren 75mg SR tab Apo-Diclo 100mg SR tab Novo-Difenac 100mg SR tab pms-Diclofenac 100mg SR tab Sandoz Diclofenac 100mg SR tab Voltaren 100mg SR tab APX SAS TEV PMS SDZ NVR APX TEV PMS SDZ NVR 0.3500 0.3500 0.3500 0.3500 0.3500 0.3500 02231506 02261928 00632724 02231508 02261936 00632732 02039486 02048493 pms-Diclofenac 50mg supp Sandoz Diclofenac 50mg supp Voltaren 50mg supp pms-Diclofenac 100mg supp Sandoz Diclofenac 100mg supp Voltaren 100mg supp Apo-Diflunisal 250mg tab Novo-Diflunisal 250mg tab PMS SDZ NVR PMS SDZ NVR APX TEV 02039494 02241163 02230997 02097249 02370611 02242538 02355752 02229781 02243338 Apo-Diflunisal 500mg tab Dihydroergotamine 1mg/mL inj Apo-Diltiaz 120mg CD cap Cardizem 120mg CD cap CO Diltiazem CD 120mg cap Novo-Diltazem 120mg CD cap pms-Diltiazem CD 120mg cap ratio-Diliazem 120mg CD cap (discontinued) Sandoz Diltiazem 120mg CD cap APX SDZ APX BVL COB TEV PMS TEV SDZ diazepam 5mg/mL inj diclofenac sodium 25mg EC tab diclofenac sodium 100mg SR tab diclofenac sodium 50mg supp diclofenac sodium 100mg supp diflunisal 250mg tab diflunisal 500mg tab dihydroergotamine 1mg/mL inj diltiazem 120mg CD cap 0.5788 0.5788 0.5788 0.5788 0.5788 0.4670 0.4670 0.4670 0.6286 0.6286 0.6286 0.1750 0.1750 0.3500 3.7933 0.5174 0.5174 0.5174 0.5174 0.5174 0.5174 0.5174 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 22 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength diltiazem 180mg CD cap DIN 02230998 02097257 02370638 02242539 02355760 02229782 02243339 Brand Apo-Diltiaz 180mg CD cap Cardizem 180mg CD cap CO Diltiazem CD 180mg cap Novo-Diltazem 180mg CD cap pms-Diltiazem CD 180mg cap ratio-Diliazem 180mg CD cap (discontinued) Sandoz Diltiazem 180mg CD cap MFR MRP APX 0.6869 BVL 0.6869 COB 0.6869 TEV 0.6869 PMS 0.6869 TEV 0.6869 SDZ 0.6869 diltiazem 240mg CD cap 02230999 02097265 02370646 02242540 02355779 02229783 02243340 02229526 02097273 02370654 02242541 02355787 02229784 02243341 02291037 02370441 02271605 02245918 02231150 Apo-Diltiaz 240mg CD cap Cardizem 240mg CD cap CO Diltiazem CD 240mg cap Novo-Diltazem 240mg CD cap pms-Diltiazem CD 240mg cap ratio-Diliazem 240mg CD cap (discontinued) Sandoz Diltiazem 240mg CD cap Apo-Diltiaz 300mg CD cap Cardizem 300mg CD cap CO Diltiazem CD 300mg cap Novo-Diltazem 300mg CD cap pms-Diltiazem CD 300mg cap ratio-Diliazem 300mg CD cap (discontinued) Sandoz Diltiazem 300mg CD cap Apo-Diltiaz TZ 120mg ER cap CO Diltiazem T 120mg cap Novo-Diltiazem HCL 120mg ER cap Sandoz Diltiazem T 120mg ER cap Tiazac 120mg ER cap APX BVL COB TEV PMS TEV SDZ APX BVL COB TEV PMS TEV SDZ APX COB TEV SDZ BVL 0.9111 0.9111 0.9111 0.9111 0.9111 0.9111 0.9111 1.1388 1.1388 1.1388 1.1388 1.1388 1.1388 1.1388 0.2987 0.2987 0.2987 0.2987 0.2987 02291045 02370492 02271613 02245919 02231151 02291053 02370506 02271621 02245920 02231152 02291061 02370514 02271648 02245921 02231154 02291088 02370522 02271656 02245922 Apo-Diltiaz TZ 180mg ER cap CO Diltiazem T 180mg cap Novo-Diltiazem HCL 180mg ER cap Sandoz Diltiazem T 180mg ER cap Tiazac 180mg ER cap Apo-Diltiaz TZ 240mg ER cap CO Diltiazem T 240mg cap Novo-Diltiazem HCL 240mg ER cap Sandoz Diltiazem T 240mg ER cap Tiazac 240mg ER cap Apo-Diltiaz TZ 300mg ER cap CO Diltiazem T 300mg cap Novo-Diltiazem HCL 300mg ER cap Sandoz Diltiazem T 300mg ER cap Tiazac 300mg ER cap Apo-Diltiaz TZ 360mg ER cap CO Diltiazem T 360mg cap Novo-Diltiazem HCL 360mg ER cap Sandoz Diltiazem T 360mg ER cap APX COB TEV SDZ BVL APX COB TEV SDZ BVL APX COB TEV SDZ BVL APX COB TEV SDZ 0.4045 0.4045 0.4045 0.4045 0.4045 0.5365 0.5365 0.5365 0.5365 0.5365 0.6607 0.6607 0.6607 0.6607 0.6607 0.8089 0.8089 0.8089 0.8089 diltiazem 300mg CD cap diltiazem 120mg ER cap diltiazem 180mg ER cap diltiazem 240mg ER cap diltiazem 300mg ER cap diltiazem 360mg ER cap Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 23 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength diltiazem 360mg ER cap DIN 02231155 Tiazac 360mg ER cap MFR MRP BVL 0.8089 diltiazem HCl 30mg tab 00771376 00862924 Apo-Diltiaz 30mg tab Novo-Diltazem 30mg tab APX TEV 0.1866 0.1866 diltiazem HCl 60mg tab 00771384 00862932 00392731 Apo-Diltiaz 60mg tab Novo-Diltazem 60mg tab Dimenhydrinate 10mg/mL IV amp APX TEV SDZ 0.3273 0.3273 0.3520 00392537 00013579 02243231 00493392 Dimenhydrinate 50mg/mL IM inj Gravol I 50mg/mL IM inj Dimethyl Sulfoxide 50% irr sol Rimso 50% irr sol SDZ CHU SDZ BCH 1.1500 1.1500 1.1840 1.1840 dipyridamole 25mg tab 00895644 Apo-Dipyridamole-FC 25mg tab APX 0.2633 dipyridamole 50mg tab dipyridamole 75mg tab divalproex sodium 125mg tab 00895652 00895660 Apo-Dipyridamole-FC 50mg tab Apo-Dipyridamole-FC 75mg tab APX APX 0.2932 0.4397 02239698 00596418 02239701 Apo-Divalproex 125mg tab Epival 125mg tab Novo-Divalproex 125mg tab APX ABB TEV 0.1032 0.1032 0.1032 divalproex sodium 250mg tab 02239699 00596426 02239702 02239700 00596434 02239703 Apo-Divalproex 250mg tab Epival 250mg tab Novo-Divalproex 250mg tab Apo-Divalproex 500mg tab Epival 500mg tab Novo-Divalproex 500mg tab APX ABB TEV APX ABB TEV 0.1855 0.1855 0.1855 0.3711 0.3711 0.3711 02242010 02103613 02350440 02369206 02278669 02236466 02268078 01912070 02157195 02316307 02216205 02299615 Dobutamine 12.5mg/mL inj Apo-Domperidone 10mg tab Domperidone 10mg tab Jamp-Domperidone 10mg tab MYLAN-Domperidone 10mg tab pms-Domperidone 10mg tab RAN-Domperidone 10mg tab ratio-Domperidone 10mg tab Teva-Domperidone 10mg tab Sandoz Dorzolamide 2% oph sol Trusopt 2% oph sol Apo-Dorzo-Timop 2%/0.5% oph sol SDZ APX SAS JPC MYL PMS RAN TEV TEV SDZ MSD APX 1.4885 0.0832 0.0832 0.0832 0.0832 0.0832 0.0832 0.0832 0.0832 1.3279 1.3279 2.0097 02240113 02344351 02320525 02240588 01958100 02240498 02242728 02244527 02240589 Cosopt 2%/0.5% oph sol Sandoz Dorzolamide/Timolol 2%/0.5% oph sol Teva-Dorzotimol 2%/0.5% oph sol Apo-Doxazosin 1mg tab Cardura-1 1mg tab MYLAN-Doxazosin 1mg tab Novo-Doxazosin 1mg tab pms-Doxazosin 1mg tab Apo-Doxazosin 2mg tab MSD SDZ TEV APX PFI MYL TEV PMS APX 2.0097 2.0097 2.0097 0.1989 0.1989 0.1989 0.1989 0.1989 0.2385 dimenhydrinate 10mg/mL IV inj dimenhydrinate 50mg/mL IM inj dimethyl sulfoxide 500mg/g (50%) irr sol divalproex sodium 500mg tab dobutamine 12.5mg/mL inj domperidone maleate 10mg tab dorzolamide HCI 2% oph sol dorzolamide HCI 2% & timolol maleate 0.5% oph sol doxazosin 1mg tab doxazosin 2mg tab Brand Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 24 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength doxazosin 2mg tab DIN 01958097 02240499 02242729 02244528 Brand Cardura-2 2mg tab MYLAN-Doxazosin 2mg tab Novo-Doxazosin 2mg tab pms-Doxazosin 2mg tab MFR MRP PFI 0.2385 MYL 0.2385 TEV 0.2385 PMS 0.2385 doxazosin 4mg tab 02240590 01958119 02240500 02242730 02244529 Apo-Doxazosin 4mg tab Cardura-4 4mg tab MYLAN-Doxazosin 4mg tab Novo-Doxazosin 4mg tab pms-Doxazosin 4mg tab APX PFI MYL TEV PMS 0.3102 0.3102 0.3102 0.3102 0.3102 doxepin HCl 10mg cap 02049996 00024325 Apo-Doxepin 10mg cap Sinequan 10mg cap APX ERF 0.1889 0.1889 doxepin HCl 25mg cap 02050005 01913425 00024333 02050013 01913433 00024341 Apo-Doxepin 25mg cap Novo-Doxepin 25mg cap Sinequan 25mg cap Apo-Doxepin 50mg cap Novo-Doxepin 50mg cap Sinequan 50mg cap APX TEV ERF APX TEV ERF 0.2140 0.2140 0.2140 0.2923 0.2923 0.2923 02050021 01913441 00400750 02050048 01913468 00326925 01913476 Apo-Doxepin 75mg cap Novo-Doxepin 75mg cap Sinequan 75mg cap Apo-Doxepin 100mg cap Novo-Doxepin 100mg cap Sinequan 100mg cap Novo-Doxepin 150mg cap APX TEV ERF APX TEV ERF TEV 0.4302 0.4302 0.4302 0.5160 0.5160 0.5160 1.1507 00740713 02351234 00725250 00024368 Apo-Doxy 100mg cap Doxycycline 100mg cap Novo-Doxylin 100mg cap Vibramycin 100mg cap APX SAS TEV PFI 0.5949 0.5949 0.5949 0.5949 doxycycline 100mg tab (Vibra-tabs) 00874256 02351242 02158574 Apo-Doxy 100mg tab Doxycycline 100mg tab Novo-Doxylin 100mg tab APX SAS TEV 0.5860 0.5860 0.5860 enalapril 2.5mg tab 02020025 02291878 02300036 02300680 02352230 02299933 00851795 Apo-Enalapril 2.5mg tab CO Enalapril 2.5mg tab MYLAN-Enalapril 2.5mg tab Novo-Enalapril 2.5mg tab RAN-Enalapril 2.5mg tab Sandoz Enalapril 2.5mg tab Vasotec 2.5mg tab APX COB MYL TEV RAN SDZ FRS 0.2737 0.2737 0.2737 0.2737 0.2737 0.2737 0.2737 enalapril 5mg tab 02019884 02291886 02300044 02233005 02352249 02299941 Apo-Enalapril 5mg tab CO Enalapril 5mg tab MYLAN-Enalapril 5mg tab Novo-Enalapril 5mg tab RAN-Enalapril 5mg tab Sandoz Enalapril 5mg tab APX COB MYL TEV RAN SDZ 0.3239 0.3239 0.3239 0.3239 0.3239 0.3239 doxepin HCl 50mg cap doxepin HCl 75mg cap doxepin HCl 100mg cap doxepin HCl 150mg cap doxycycline 100mg cap Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 25 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength enalapril 5mg tab DIN 00708879 Vasotec 5mg tab MFR MRP FRS 0.3239 enalapril 10mg tab 02019892 02291894 02300052 02233006 02352257 02299968 00670901 Apo-Enalapril 10mg tab CO Enalapril 10mg tab MYLAN-Enalapril 10mg tab Novo-Enalapril 10mg tab RAN-Enalapril 10mg tab Sandoz Enalapril 10mg tab Vasotec 10mg tab APX COB MYL TEV RAN SDZ FRS 0.3891 0.3891 0.3891 0.3891 0.3891 0.3891 0.3891 enalapril 20mg tab 02019906 02291908 02300060 02233007 02352265 02299976 00670928 02352923 Apo-Enalapril 20mg tab CO Enalapril 20mg tab MYLAN-Enalapril 20mg tab Novo-Enalapril 20mg tab RAN-Enalapril 20mg tab Sandoz Enalapril 20mg tab Vasotec 20mg tab Apo-Enalapril Maleate/HCTZ 5/12.5mg tab APX COB MYL TEV RAN SDZ FRS APX 0.4696 0.4696 0.4696 0.4696 0.4696 0.4696 0.4696 0.4941 02300222 Novo-Enalapril/HCTZ 5/12.5mg tab TEV 0.4941 02352931 Apo-Enalapril Maleate/HCTZ 10/25mg tab APX 0.6108 02300230 00657298 Novo-Enalapril/HCTZ 10/25mg tab Vaseretic 10/25mg tab TEV FRS 0.6108 0.6108 entacapone 200mg tab (exception status) 02243763 02390337 02380005 02375559 Comtan 200mg tab MYLAN-Entacapone 200mg tab Sandoz Entacapone 200mg tab Teva-Entacapone 200mg tab NVR MYL SDZ TEV 0.5687 0.5687 0.5687 0.5687 entecavir 0.5mg tab (exception status) 02396955 02282224 00726672 00607142 Apo-Entecavir 0.5mg tab Baraclude 0.5mg tab Apo-Erythro-EC 250mg cap ERYC 250mg cap APX BRI AAP PFI 16.5000 16.5000 0.4232 0.4232 01925938 00873454 00637416 Apo-Erythro-EC 333mg cap ERYC 333mg cap Erythro-ES 600mg tab AAP PFI AAP 0.4700 0.4700 0.3649 00605859 Novo-Rythro EES 200mg/5mL susp TEV 0.0923 00652318 02231583 Novo-Rythro EES 400mg/5mL susp Eprex 1,000iu/0.5mL syringe inj TEV JAN 0.1398 02231584 Eprex 2,000iu/0.5mL syringe inj JAN 61.8450 02231585 Eprex 3,000iu/0.3mL syringe inj JAN 154.6125 02231586 Eprex 4,000iu/0.4mL syringe inj JAN 154.6125 02243400 Eprex 5,000iu/0.5mL syringe inj JAN 154.6125 enalapril 5mg & hydrochlorothiazide 12.5mg tab enalapril 10mg & hydrochlorothiazide 25mg tab erythromycin base 250mg cap erythromycin base 333mg cap erythromycin ethylsuccinate 600mg tab erythromycin ethylsuccinate 40mg/mL o/l erythromycin ethylsuccinate 80mg/mL o/l erythropoeietin 2,000iu/mL inj (exception status) erythropoeietin 4,000iu/mL inj (exception status) erythropoeietin 10,000iu/mL inj (exception status) erythropoeietin 10,000iu/mL inj (exception status) erythropoeietin 10,000iu/mL inj (exception status) Brand Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 26 of 87 PRP 30.9225 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 1 Generic Name and Strength erythropoeietin 10,000iu/mL inj (exception status) erythropoeietin 10,000iu/mL inj (exception status) erythropoeietin 10,000iu/mL inj (exception status) erythropoeietin 40,000iu/mL inj (exception status) erythropoeietin 40,000iu/mL inj (exception status) erythropoeietin 40,000iu/mL inj (exception status) estradiol 50mcg/day patch (exception status) 2 DIN 02243401 Brand Eprex 6,000iu/0.6mL syringe inj MFR MRP JAN PRP 154.6125 02243403 Eprex 8,000iu/0.8mL syringe inj JAN 154.6125 02231587 Eprex 10,000iu/mL syringe inj JAN 154.6125 02243239 Eprex 20,000iu/0.5mL syringe inj JAN 600.5258 02288680 Eprex 30,000iu/0.75mL syringe inj JAN 600.5258 02240722 Eprex 40,000iu/mL syringe inj JAN 450.3944 02244000 Estradot 50mcg/day patch NVR 2.4125 02246967 Sandoz Estradiol Derm 50mcg/day patch SDZ 2.4125 02244001 Estradot 75mcg/day patch NVR 2.5875 02246968 Sandoz Estradiol Derm 75mcg/day patch SDZ 2.5875 02244002 Estradot 100mcg/day patch NVR 2.7375 02246969 02242903 Sandoz Estradiol Derm 100mcg/day patch Enbrel 25mg Pdr for inj SDZ AGA 2.7375 02274728 02248686 02245330 02263866 Enbrel 50mg/mL inj CO Etidronate 200mg tab MYLAN-Etidronate 200mg tab CO Etidrocal sequential kit AGA COB 0.4997 MYL 0.4997 COB 19.9900 02176017 02353210 02247323 02324199 Didrocal sequential kit Etidrocal kit MYLAN-Eti-Cal carepac Novo-Etidronatecal kit WNC SAS MYL TEV etodolac 200mg cap 02232317 Etodolac 200mg cap AAP etodolac 300mg cap 02232318 02369257 02339501 02339528 02242705 02390183 02292025 02305682 02229110 02278081 02278634 02292041 02305690 Etodolac 300mg cap Afinitor 2.5mg tab Afinitor 5mg tab Afinitor 10mg tab Aromasin 25mg tab CO Exemestane 25mg tab Apo-Famciclovir 125mg tab CO Famciclovir 125mg tab Famvir 125mg tab pms-Famciclovir 125mg tab Sandoz Famciclovir 125mg tab Apo-Famciclovir 250mg tab CO Famciclovir 250mg tab AAP NVR NVR NVR PFI COB APX COB NVR PMS SDZ APX COB estradiol 75mcg/day patch (exception status) estradiol 100mcg/day patch (exception status) etanercept 25mg powder for inj (exception status) etanercept 50mg/mL inj (exception status) etidronate 200mg tab etidronic disodium 400mg & calcium carbonate 500mg tab, sequential kit (exception status) everolimus 2.5mg tab (exception status) everolimus 5mg tab (exception status) everolimus 10mg tab (exception status) exemestane 25mg tab famciclovir 125mg tab famciclovir 250mg tab 210.7558 19.9900 19.9900 19.9900 19.9900 0.3500 0.3500 201.8100 201.8100 201.8100 3.9008 3.9008 1.3940 1.3940 1.3940 1.3940 1.3940 1.8733 1.8733 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 27 of 87 421.6364 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength famciclovir 250mg tab DIN 02229129 02278103 02278642 Brand Famvir 250mg tab pms-Famciclovir 250mg tab Sandoz Famciclovir 250mg tab MFR MRP NVR 1.8733 PMS 1.8733 SDZ 1.8733 famciclovir 500mg tab 02292068 02305704 02177102 02278111 02278650 Apo-Famciclovir 500mg tab CO Famciclovir 500mg tab Famvir 500mg tab pms-Famciclovir 500mg tab Sandoz Famciclovir 500mg tab APX COB NVR PMS SDZ famotidine 20mg tab 01953842 02351102 02196018 02022133 00710121 Apo-Famotidine 20mg tab Famotidine 20mg tab MYLAN-Famotidine 20mg tab Novo-Famotidine 20mg tab Pepcid 20mg tab APX SAS MYL TEV FRS 0.1800 0.1800 0.1800 0.1800 0.1800 famotidine 40mg tab 01953834 02351110 02196026 02022141 00710113 Apo-Famotidine 40mg tab Famotidine 40mg tab MYLAN-Famotidine 40mg tab Novo-Famotidine 40mg tab Pepcid 40mg tab (discontinued) APX SAS MYL TEV FRS 0.3600 0.3600 0.3600 0.3600 0.3600 famotidine 10mg/mL inj 02247745 Famotidine Omega 10mg/mL inj HOS 1.9850 famotidine 10mg/mL inj (pf) 02247735 00851779 02280264 Famotidine Omega (PF) 10mg/mL inj Plendil 5mg tab Sandoz Felodipine 5mg tab HOS AZE SDZ 1.9850 0.4620 0.4620 felodipine 10mg tab (Plendil) 00851787 02280272 Plendil 10mg tab Sandoz Felodipine 10mg tab AZE SDZ 0.6733 0.6733 felodipine 5mg tab (Renedil) 02221993 02280264 Renedil 5mg tab Sandoz Felodipine 5mg tab SAV SDZ 0.4620 0.4620 felodipine 10mg tab (Renedil) 02222000 02280272 Renedil 10mg tab Sandoz Felodipine 10mg tab SAV SDZ 0.6733 0.6733 fenofibrate 67mg cap 02243180 02243551 Apo-Feno-Micro 67mg cap Novo-Fenofibrate Micronized 67mg cap APX TEV 0.4325 0.4325 fenofibrate 100mg tab 02246859 02356570 02241601 02289083 02288044 Apo-Feno-Super 100mg tab Fenofibrate-S 100mg tab Lipidil Supra 100mg tab Novo-Fenofibrate S 100mg tab Sandoz Fenofibrate S 100mg tab APX SAS SPH TEV SDZ 0.5407 0.5407 0.5407 0.5407 0.5407 fenofibrate 160mg tab 02246860 02356589 02241602 02289091 02288052 Apo-Feno-Super 160mg tab Fenofibrate-S 160mg tab Lipidil Supra 160mg tab Novo-Fenofibrate S 160mg tab Sandoz Fenofibrate S 160mg tab APX SAS SPH TEV SDZ 0.4362 0.4362 0.4362 0.4362 0.4362 fenofibrate 200mg cap 02239864 02286092 02146959 02240210 Apo-Feno-Micro 200mg cap Fenofibrate Micro 200mg cap Lipidil Micro 200mg cap MYLAN-Fenofibrate Micro 200mg cap APX SAS SPH MYL 0.3812 0.3812 0.3812 0.3812 felodipine 5mg tab (Plendil) 2.4260 2.4260 2.4260 2.4260 2.4260 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 28 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength fenofibrate 200mg cap DIN 02243552 02273551 02250039 Brand Novo-Fenofibrate Micronized 200mg cap pms-Fenofibrate Micro 200mg cap ratio-Fenofibrate MC 200mg cap MFR MRP TEV 0.3812 PMS 0.3812 TEV 0.3812 fentanyl 12mcg/hr patch (exception status) 02396696 MYLAN-Fentanyl Matrix 12mcg/hr patch MYL 2.2300 fentanyl 25mcg/hr patch (exception status) 02341379 02330105 02311925 02327112 02275813 pms-Fentanyl MTX 12mcg/hr patch RAN-Fentanyl MTX 12mcg/hr patch ratio-Fentanyl 12mcg/hr patch Sandoz Fentanyl 12mcg/hr patch Duragesic MAT 25mcg/hr patch PMS RAN TEV SDZ JAN 2.2300 2.2300 2.2300 2.2300 4.0236 fentanyl 50mcg/hr patch (exception status) 02396718 02314630 02341387 02330113 02282941 02327120 02275821 MYLAN-Fentanyl Matrix 25mcg/hr patch Novo-Fentanyl 25mcg/hr patch pms-Fentanyl MTX 25mcg/hr patch RAN-Fentanyl MTX 25mcg/hr patch ratio-Fentanyl 25mcg/hr patch Sandoz Fentanyl 25mcg/hr patch Duragesic MAT 50mcg/hr patch MYL TEV PMS RAN TEV SDZ JAN 4.0236 4.0236 4.0236 4.0236 4.0236 4.0236 7.5719 fentanyl 75mcg/hr patch (exception status) 02396726 02314649 02341395 02330121 02282968 02327147 02275848 MYLAN-Fentanyl Matrix 50mcg/hr patch Novo-Fentanyl 50mcg/hr patch pms-Fentanyl MTX 50mcg/hr patch RAN-Fentanyl MTX 50mcg/hr patch ratio-Fentanyl 50mcg/hr patch Sandoz Fentanyl 50mcg/hr patch Duragesic MAT 75mcg/hr patch MYL 7.5719 TEV 7.5719 PMS 7.5719 RAN 7.5719 TEV 7.5719 SDZ 7.5719 JAN 10.6498 02396734 02314657 02341409 02330148 02282976 02327155 MYLAN-Fentanyl Matrix 75mcg/hr patch Novo-Fentanyl 75mcg/hr patch pms-Fentanyl MTX 75mcg/hr patch RAN-Fentanyl MTX 75mcg/hr patch ratio-Fentanyl 75mcg/hr patch Sandoz Fentanyl 75mcg/hr patch MYL TEV PMS RAN TEV SDZ 10.6498 10.6498 10.6498 10.6498 10.6498 10.6498 02275856 Duragesic MAT 100mcg/hr patch JAN 13.2559 02396742 02314665 02341417 02330156 02282984 02327163 02365383 02354462 02355043 02357224 02389878 MYLAN-Fentanyl Matrix 100mcg/hr patch Novo-Fentanyl 100mcg/hr patch pms-Fentanyl MTX 100mcg/hr patch RAN-Fentanyl MTX 100mcg/hr patch ratio-Fentanyl 100mcg/hr patch Sandoz Fentanyl 100mcg/hr patch Apo-Finasteride 5mg tab CO Finasteride 5mg tab Finasteride 5mg tab Jamp-Finasteride 5mg tab MINT-Finasteride 5mg tab MYL TEV PMS RAN TEV SDZ APX COB AHC JPC MNT 13.2559 13.2559 13.2559 13.2559 13.2559 13.2559 0.6531 0.6531 0.6531 0.6531 0.6531 fentanyl 100mcg/hr patch (exception status) finasteride 5mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 29 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength finasteride 5mg tab DIN 02356058 02348500 02310112 02010909 02371820 02306905 02322579 Brand MYLAN-Finasteride 5mg tab Novo-Finasteride 5mg tab pms-Finasteride 5mg tab Proscar 5mg tab RAN-Finasteride 5mg tab ratio-Finasteride 5mg tab Sandoz Finasteride 5mg tab MFR MRP MYL 0.6531 TEV 0.6531 PMS 0.6531 FRS 0.6531 RAN 0.6531 TEV 0.6531 SDZ 0.6531 flecainide 50mg tab 02275538 01966197 Flecainide 50mg tab Tambocor 50mg tab AAP MDS 0.4292 0.4292 flecainide 100mg tab 02275546 01966200 Flecainide 100mg tab Tambocor 100mg tab AAP MDS 0.8585 0.8585 floctafenine 200mg tab floctafenine 400mg tab 02244680 02244681 Floctafenine 200mg tab Floctafenine 400mg tab AAP AAP fluconazole 50mg tab 02237370 02281260 02245292 02236978 02245643 02237371 02281279 02245293 02236979 02245644 02241895 02282348 02246082 Apo-Fluconazole 50mg tab CO Fluconazole 50mg tab MYLAN-Fluconazole 50mg tab Novo-Fluconazole 50mg tab pms-Fluconazole 50mg tab Apo-Fluconazole 100mg tab CO Fluconazole 100mg tab MYLAN-Fluconazole 100mg tab Novo-Fluconazole 100mg tab pms-Fluconazole 100mg tab Apo-Fluconazole 150mg cap pms-Fluconazole 150mg cap Flunarizine 5mg cap APX COB MYL TEV PMS APX COB MYL TEV PMS APX PMS AAP 1.8066 1.8066 1.8066 1.8066 1.8066 3.2048 3.2048 3.2048 3.2048 3.2048 7.0725 7.0725 0.7817 02161923 00716863 Lidex 0.05% cr Lyderm 0.05% cr VAL TPH 0.2444 0.2444 fluocinonide 0.05% gel 02236997 02161974 Lyderm 0.05% gel Topsyn 0.05% gel TPH MDS 0.3418 0.3418 fluocinonide 0.05% oint 02161966 02236996 00247855 00432814 02216353 02385627 02242177 02286068 02380560 02237813 02223481 02177579 02018985 02241371 Lidex 0.05% oint Lyderm 0.05% oint FML Liquifilm 0.1% oph susp Sandoz Fluorometholone 0.1% oph susp Apo-Fluoxetine 10mg cap Auro-Fluoxetine 10mg cap CO Fluoxetine 10mg cap Fluoxetine 10mg cap MINT-Fluoxetine 10mg cap MYLAN-Fluoxetine 10mg cap phl-Fluoxetine 10mg cap pms-Fluoxetine 10mg cap Prozac 10mg cap ratio-Fluoxetine 10mg cap VAL TPH ALL SDZ APX ARO COB SAS MNT MYL PHL PMS LIL TEV 0.3370 0.3370 1.7880 1.7880 0.8650 0.8650 0.8650 0.8650 0.8650 0.8650 0.8650 0.8650 0.8650 0.8650 fluconazole 100mg tab fluconazole 150mg cap flunarizine 5mg cap fluocinonide 0.05% cr fluorometholone 0.1% oph susp fluoxetine 10mg cap 0.1167 0.2333 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 30 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength fluoxetine 10mg cap DIN 02243486 02216582 02302659 Brand Sandoz Fluoxetine 10mg cap Teva-Fluoxetine 10mg cap Zym-Fluoxetine 10mg cap MFR MRP SDZ 0.8650 TEV 0.8650 ZYM 0.8650 fluoxetine 20mg cap 02216361 02385635 02242178 02286076 02383241 02386402 02380579 02237814 02223503 02177587 00636622 02241374 02243487 02216590 02302667 Apo-Fluoxetine 20mg cap Auro-Fluoxetine 20mg cap CO Fluoxetine 20mg cap Fluoxetine 20mg cap Fluoxetine BP 20mg cap Jamp-Fluoxetine 20mg cap MINT-Fluoxetine 20mg cap MYLAN-Fluoxetine 20mg cap phl-Fluoxetine 20mg cap pms-Fluoxetine 20mg cap Prozac 20mg cap ratio-Fluoxetine 20mg cap Sandoz Fluoxetine 20mg cap Teva-Fluoxetine 20mg cap Zym-Fluoxetine 20mg cap APX ARO COB SAS AHI JPC MNT MYL PHL PMS LIL TEV SDZ TEV ZYM flurbiprofen 50mg tab 00647942 01912046 02100509 00600792 01912038 02100517 Ansaid 50mg tab (discontinued) Apo-Flurbiprofen 50mg tab Novo-Flurprofen 50mg tab Ansaid 100mg tab (discontinued) Apo-Flurbiprofen 100mg tab Novo-Flurprofen 100mg tab PFI APX TEV PFI APX TEV 0.3039 0.3039 0.3039 flutamide 250mg tab 02238560 00637726 02230089 02230104 Apo-Flutamide 250mg tab Euflex 250mg tab Novo-Flutamide 250mg tab pms-Flutamide 250mg tab APX SCH TEV PMS 1.3530 1.3530 1.3530 1.3530 fluvastatin 20mg cap 02061562 02299224 Lescol 20mg cap Teva-Fluvastatin 20mg cap NVR TEV 0.7048 0.7048 fluvastatin 40mg cap 02061570 02299232 Lescol 40mg cap Teva-Fluvastatin 40mg cap NVR TEV 0.9896 0.9896 fluvoxamine 50mg tab 02231329 02255529 01919342 02239953 02240682 02218453 02231330 02255537 01919369 02239954 02240683 02218461 Apo-Fluvoxamine 50mg tab CO Fluvoxamine 50mg tab Luvox 50mg tab Novo-Fluvoxamine 50mg tab pms-Fluvoxamine 50mg tab ratio-Fluvoxamine 50mg tab Apo-Fluvoxamine 100mg tab CO Fluvoxamine 100mg tab Luvox 100mg tab Novo-Fluvoxamine 100mg tab pms-Fluvoxamine 100mg tab ratio-Fluvoxamine 100mg tab APX COB SPH TEV PMS TEV APX COB SPH TEV PMS TEV 0.3000 0.3000 0.3000 0.3000 0.3000 0.3000 0.5392 0.5392 0.5392 0.5392 0.5392 0.5392 flurbiprofen 100mg tab fluvoxamine 100mg tab 0.6438 0.6438 0.6438 0.6438 0.6438 0.6438 0.6438 0.6438 0.6438 0.6438 0.6438 0.6438 0.6438 0.6438 0.6438 0.1750 0.1750 0.1750 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 31 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength fosinopril 10mg tab DIN 02266008 02331004 01907107 02262401 02294524 02247802 Brand Apo-Fosinopril 10mg tab Jamp-Fosinopril 10mg tab Monopril 10mg tab MYLAN-Fosinopril 10mg tab RAN-Fosinopril 10mg tab Teva-Fosinopril 10mg tab MFR MRP APX 0.3049 JPC 0.3049 BRI 0.3049 MYL 0.3049 RAN 0.3049 TEV 0.3049 fosinopril 20mg tab 02266016 02331012 01907115 02262428 02294532 02247803 00396788 02351420 02224690 00337730 Apo-Fosinopril 20mg tab Jamp-Fosinopril 20mg tab Monopril 20mg tab MYLAN-Fosinopril 20mg tab RAN-Fosinopril 20mg tab Teva-Fosinopril 20mg tab Apo-Furosemide 20mg tab Furosemide 20mg tab Lasix 20mg tab (discontinued) Novo-Semide 20mg tab APX JPC BRI MYL RAN TEV APX SAS SAV TEV 0.3666 0.3666 0.3666 0.3666 0.3666 0.3666 0.0373 0.0373 0.0373 0.0373 furosemide 40mg tab 00362166 02351439 02224704 00337749 Apo-Furosemide 40mg tab Furosemide 40mg tab Lasix 40mg tab Novo-Semide 40mg tab APX SAS SAV TEV 0.0558 0.0558 0.0558 0.0558 furosemide 80mg tab 00707570 02351447 00765953 Apo-Furosemide 80mg tab Furosemide 80mg tab Novo-Semide 80mg tab APX SAS TEV 0.1220 0.1220 0.1220 gabapentin 100mg cap 02244304 02321203 02256142 02353245 02285819 02361469 02248259 02084260 02246314 02243446 02319055 02244513 02244305 02321211 02256150 02353253 02285827 02361485 02248260 02084279 02246315 02243447 Apo-Gabapentin 100mg cap Auro-Gabapentin 100mg cap CO Gabapentin 100mg cap Gabapentin 100mg cap GD-Gabapentin 100mg cap Jamp-Gabapentin 100mg cap MYLAN-Gabapentin 100mg cap Neurontin 100mg cap phl-Gabapentin 100mg cap pms-Gabapentin 100mg cap RAN-Gabapentin 100mg cap Teva-Gabapentin 100mg cap Apo-Gabapentin 300mg cap Auro-Gabapentin 300mg cap CO Gabapentin 300mg cap Gabapentin 300mg cap GD-Gabapentin 300mg cap Jamp-Gabapentin 300mg cap MYLAN-Gabapentin 300mg cap Neurontin 300mg cap phl-Gabapentin 300mg cap pms-Gabapentin 300mg cap APX ARO COB SAS GMD JPC MYL PFI PHL PMS RAN TEV APX ARO COB SAS GMD JPC MYL PFI PHL PMS 0.1460 0.1460 0.1460 0.1460 0.1460 0.1460 0.1460 0.1460 0.1460 0.1460 0.1460 0.1460 0.3553 0.3553 0.3553 0.3553 0.3553 0.3553 0.3553 0.3553 0.3553 0.3553 furosemide 20mg tab gabapentin 300mg cap Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 32 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength gabapentin 300mg cap DIN 02319063 02244514 Brand RAN-Gabapentin 300mg cap Teva-Gabapentin 300mg cap MFR MRP RAN 0.3553 TEV 0.3553 gabapentin 400mg cap 02244306 02321238 02256169 02353261 02285835 02361493 02248261 02084287 02246316 02243448 02319071 02260905 02244515 Apo-Gabapentin 400mg cap Auro-Gabapentin 400mg cap CO Gabapentin 400mg cap Gabapentin 400mg cap GD-Gabapentin 400mg cap Jamp-Gabapentin 400mg cap MYLAN-Gabapentin 400mg cap Neurontin 400mg cap phl-Gabapentin 400mg cap pms-Gabapentin 400mg cap RAN-Gabapentin 400mg cap ratio-Gabapentin 400mg cap Teva-Gabapentin 400mg cap APX ARO COB SAS GMD JPC MYL PFI PHL PMS RAN TEV TEV 0.4233 0.4233 0.4233 0.4233 0.4233 0.4233 0.4233 0.4233 0.4233 0.4233 0.4233 0.4233 0.4233 gabapentin 600mg tab 02293358 02285843 02239717 02248457 02293366 02285851 02239718 02247346 Apo-Gabapentin 600mg tab GD-Gabapentin 600mg tab Neurontin 600mg tab Teva-Gabapentin 600mg tab Apo-Gabapentin 800mg tab GD-Gabapentin 800mg tab Neurontin 800mg tab Teva-Gabapentin 800mg tab APX GMD PFI TEV APX GMD PFI TEV 0.6350 0.6350 0.6350 0.6350 0.8467 0.8467 0.8467 0.8467 02339439 MYLAN-Galantamine ER 8mg cap MYL 1.7451 02316943 02266717 02377950 PAT-Galantamine ER 8mg cap Reminyl ER 8mg cap Teva-Galantamine ER 8mg cap PPH JAN TEV 1.7451 1.7451 1.7451 02339447 MYLAN-Galantamine ER 16mg cap MYL 1.7451 02316951 02266725 02377969 PAT-Galantamine ER 16mg cap Reminyl ER 16mg cap Teva-Galantamine ER 16mg cap PPH JAN TEV 1.7451 1.7451 1.7451 galantamine 24mg ER cap (exception status) 02339455 MYLAN-Galantamine ER 24mg cap MYL 1.7451 gemfibrozil 300mg cap 02316978 02266733 02377977 01979574 02185407 02241704 02239951 PAT-Galantamine ER 24mg cap Reminyl ER 24mg cap Teva-Galantamine ER 24mg cap Apo-Gemfibrozil 300mg cap MYLAN-Gemfibrozil 300mg cap Novo-Gemfibrozil 300mg cap pms-Gemfibrozil 300mg cap PPH JAN TEV APX MYL TEV PMS 1.7451 1.7451 1.7451 0.1717 0.1717 0.1717 0.1717 01979582 02230476 02142074 Apo-Gemfibrozil 600mg tab MYLAN-Gemfibrozil 600mg tab Novo-Gemfibrozil 600mg tab APX MYL TEV 0.5157 0.5157 0.5157 gabapentin 800mg tab galantamine 8mg ER cap (exception status) galantamine 16mg ER cap (exception status) gemfibrozil 600mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 33 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength gemfibrozil 600mg tab DIN 02230183 Brand pms-Gemfibrozil 600mg tab MFR MRP PMS 0.5157 gentamicin 40mg/mL inj 02242652 Gentamicin 40mg/mL inj SDZ 2.9650 gentamicin 0.3% otic sol gliclazide 80mg tab 02229441 02245247 00765996 02287072 02229519 02238103 Sandoz Gentamicin 0.3% otic sol (discontinued) Apo-Gliclazide 80mg tab Diamicron 80mg tab Gliclazide 80mg tab MYLAN-Gliclazide 80mg tab Novo-Gliclazide 80mg tab SDZ APX SEV SAS MYL TEV 1.0320 0.1304 0.1304 0.1304 0.1304 0.1304 gliclazide MR 30mg tab 02242987 02297795 97799824 97799823 97799814 97799815 97799962 97799963 97799497 97799496 97799748 97799749 97799702 97799703 97799465 97799464 97799459 97799460 97799564 97799829 97799827 97799597 97799596 97799770 97799458 97799583 97799584 97799582 97799580 97799581 97799976 97799977 97799982 97799983 97799985 97799986 97799475 Diamicron MR 30mg tab Gliclazide MR 30mg tab Accu-Chek Advantage (100) Accu-Chek Advantage (50) Accu-Chek AVIVA (100) Accu-Chek AVIVA (50) Accu-Chek Compact (102) Accu-Chek Compact (51) Accu-Chek Mobile BG Test Strip Cassette (100) Accu-Chek Mobile BG Test Strip Cassette (50) Ascensia Breeze 2 Disc (100) Ascensia Breeze 2 Disc (50) Ascensia Contour (100) Ascensia Contour (50) BGStar Test Strips (100) BGStar Test Strips (50) Contour NEXT BG Test Strips (100) Contour NEXT BG Test Strips (50) EZ Oracle (100) FreeStyle (100) FreeStyle (50) FreeStyle Lite (100) FreeStyle Lite (50) iTest (50) MyGlucoHealth Glucose Test Strips (50) NovaMax (100) NovaMax (50) On-Call Plus (100) On-Call Plus (25) On-Call Plus (50) OneTouch (100) OneTouch (50) OneTouch FastTake (100) OneTouch FastTake (50) OneTouch Ultra (100) OneTouch Ultra (50) OneTouch Verio Test Strips (100) SEV AAP BOM BOM BOM BOM BOM BOM BOM BOM BDD BDD BDD BDD SAV SAV BDD BDD THI MID MID MID MID AUT EHS NBM NBM ACO ACO ACO LFS LFS LFS LFS LFS LFS LFS 0.1524 0.1524 glucose testing strips Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 34 of 87 PRP 0.7400 0.7400 0.7400 0.7400 0.7400 0.7400 0.7400 0.7400 0.7400 0.7400 0.7400 0.7400 0.6750 0.7400 0.6989 0.7400 0.7381 0.7335 0.7400 0.7335 0.7400 0.6910 0.6730 0.7400 0.7400 0.6300 0.7000 0.6700 0.7381 0.7400 0.7381 0.7400 0.7381 0.7400 0.6943 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 1 Generic Name and Strength glucose testing strips DIN 97799476 97799840 97799841 97799451 97799478 97799479 97799601 97799532 97799531 97799602 97799603 Brand OneTouch Verio Test Strips (50) Precision Xtra (100) Precision Xtra (50) Rapid Response Blood Glucose Test Strip (50) Rightest GS100 Test Strips (100) Rightest GS100 Test Strips (50) Sidekick Blood Glucose TRUEtest (100) TRUEtest (50) TrueTrack (100) TrueTrack (50) MFR MRP LFS MID MID BTX BNM BNM HOM HOM HOM HOM HOM glyburide 2.5mg tab 01913654 02224550 02350459 00808733 01900927 02248008 01913670 Apo-Glyburide 2.5mg tab Diabeta 2.5mg tab Glyburide 2.5mg tab MYLAN-Glybe 2.5mg tab ratio-Glyburide 2.5mg tab Sandoz Glyburide 2.5mg tab Teva-Glyburide 2.5mg tab APX SAV SAS MYL TEV SDZ TEV glyburide 5mg tab 01913662 02224569 00720941 02350467 00808741 02236734 01900935 02248009 01913689 02308894 Apo-Glyburide 5mg tab Diabeta 5mg tab Euglucon 5mg tab (discontinued) Glyburide 5mg tab MYLAN-Glybe 5mg tab pms-Glyburide 5mg tab ratio-Glyburide 5mg tab Sandoz Glyburide 5mg tab Teva-Glyburide 5mg tab Granisetron 1mg tab APX 0.0683 SAV 0.0683 PMS 0.0683 SAS 0.0683 MYL 0.0683 PMS 0.0683 TEV 0.0683 SDZ 0.0683 TEV 0.0683 AAP 14.6475 00396796 00363685 00396818 00363677 00396826 00363669 Apo-Haloperidol 0.5mg tab Novo-Peridol 0.5mg tab Apo-Haloperidol 1mg tab Novo-Peridol 1mg tab Apo-Haloperidol 2mg tab Novo-Peridol 2mg tab APX TEV APX TEV APX TEV 0.0360 0.0360 0.0614 0.0614 0.1050 0.1050 00396834 00363650 00463698 00713449 02130297 02130300 Apo-Haloperidol 5mg tab Novo-Peridol 5mg tab Apo-Haloperidol 10mg tab Novo-Peridol 10mg tab Haloperidol LA 50mg/mL inj Haloperidol LA 100mg/mL inj APX TEV APX TEV SDZ SDZ 0.1487 0.1487 0.1330 0.1330 7.3600 14.7167 00441619 00441627 00441635 02327856 Apo-Hydralazine 10mg tab Apo-Hydralazine 25mg tab Apo-Hydralazine 50mg tab Apo-Hydro 12.5mg tab APX APX APX APX 0.1347 0.2314 0.3633 0.0322 granisetron 1 mg tab (exception status) haloperidol 0.5mg tab haloperidol 1mg tab haloperidol 2mg tab haloperidol 5mg tab haloperidol 10mg tab haloperidol LA 50mg/mL inj haloperidol LA 100mg/mL inj hydralazine HCl 10mg tab hydralazine HCl 25mg tab hydralazine HCl 50mg tab hydrochlorothiazide 12.5mg tab 0.0393 0.0393 0.0393 0.0393 0.0393 0.0393 0.0393 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 35 of 87 2 PRP 0.7400 0.7325 0.7400 0.7100 0.5580 0.5730 0.4444 0.5741 0.5742 0.3859 0.4444 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength hydrochlorothiazide 12.5mg tab DIN 02274086 Brand pms-Hydrochlorothiazide 12.5mg tab MFR MRP PMS 0.0322 hydrochlorothiazide 25mg tab 00326844 02360594 02247386 00021474 Apo-Hydro 25mg tab Hydrochlorothiazide 25mg tab pms-Hydrochlorothiazide 25mg tab Teva-Hydrochlorothiazide 25mg tab APX SAS PMS TEV 0.0257 0.0257 0.0257 0.0257 hydrochlorothiazide 50mg tab 00312800 02360608 00021482 02247387 Apo-Hydro 50mg tab Hydrochlorothiazide 50mg tab Novo-Hydrazide 50mg tab pms-Hydrochlorothiazide 50mg tab APX SAS TEV PMS 0.0358 0.0358 0.0358 0.0358 hydrochlorothiazide 100mg tab hydrochlorothiazide 50mg & amiloride HCl 5mg tab 00644552 00784400 Apo-Hydro 100mg tab Apo-Amilzide 50mg/5mg tab APX APX 0.1232 0.1293 02257378 01937219 MYLAN-Amilazide 50mg/5mg tab (discontinued) Novamilor 50mg/5mg tab MYL TEV 0.1293 0.1293 00180408 Aldactazide 25/25mg tab PFI 0.1078 00613231 00594377 Novo-Spirozine 25/25mg tab Aldactazide 50/50mg tab TEV PFI 0.1078 0.2281 00657182 Novo-Spirozine 50/50mg tab TEV 0.2281 00441775 Apo-Triazide 25/50mg tab APX 0.0608 00532657 02242485 02128446 00505773 02247691 Novo-Triamzide 25/50mg tab Sandoz Cortimyxin oph oint (discontinued) Anodan-HC 0.5% oint Anusol-HC 0.5% oint Sandoz Anuzinc HC 0.5% oint TEV SDZ ODN JNJ SDZ 0.0608 3.6143 0.4130 0.4130 0.4130 hydrocortisone 10mg supp 02236399 00476285 02242798 Anodan-HC 10mg supp Anusol-HC 10mg supp Sandoz Anuzinc HC 10mg supp ODN JNJ SDZ 0.6075 0.6075 0.6075 hydrocortisone valerate 0.2% cr hydrocortisone, framycetin sulfate & cinchocaine HCl oint 02242984 Hydroval 0.2% cr TPH 0.1212 02247322 Proctol oint ODN 0.5960 02223252 02226383 02242527 02247882 Proctosedyl oint ratio-Proctosone oint Sandoz Proctomyxin HC oint Proctol supp AXC TEV SDZ ODN 0.5960 0.5960 0.5960 0.7925 02223260 02226391 02242528 Proctosedyl supp ratio-Proctosone supp Sandoz Proctomyxin HC supp AXC TEV SDZ 0.7925 0.7925 0.7925 00505781 02234466 02247692 00476242 02240851 Anugesic-HC oint Proctodan-HC oint Sandoz Anuzinc HC Plus oint (discontinued) Anugesic-HC supp Proctodan-HC supp JNJ ODN SDZ JNJ ODN 0.7317 0.7317 0.7317 1.0875 1.0875 hydrochlorothiazide 25mg & spironolactone 25mg tab hydrochlorothiazide 50mg & spironolactone 50mg tab hydrochlorothiazide 25mg & triamterene 50mg tab hydrocortisone & antiinfectives oph oint hydrocortisone 0.5% oint hydrocortisone, framycetin sulfate & cinchocaine HCl supp hydrocortisone, pramoxine oint hydrocortisone, pramoxine supp Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 36 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength hydrocortisone, pramoxine supp DIN 02242797 Brand Sandoz Anuzinc HC Plus supp MFR MRP SDZ 1.0875 hydromorphone 2mg/mL inj 00627100 02145901 Dilaudid 2mg/mL inj Hydromorphone 2mg/mL inj PFR SDZ 1.1380 1.1380 hydromorphone 10mg/mL inj 00622133 02145928 02145936 Dilaudid HP 10mg/mL inj Hydromorphone HP 10mg/mL inj Hydromorphone HP 20mg/mL inj PFR SDZ SDZ 2.7500 2.7500 4.5100 02146126 Hydromorphone HP 50mg/mL inj SDZ 13.1500 00786535 01916386 02364115 00705438 00885444 02319403 02364123 00125083 00885436 02319411 Dilaudid 1mg/mL oral sol pms-Hydromorphone 1mg/mL oral sol Apo-Hydromorphone 1mg tab Dilaudid 1mg tab pms-Hydromorphone 1mg tab Teva-Hydromorphone 1mg tab Apo-Hydromorphone 2mg tab Dilaudid 2mg tab pms-Hydromorphone 2mg tab Teva-Hydromorphone 2mg tab PFR PMS APX PFR PMS TEV APX PFR PMS TEV 0.0665 0.0665 0.0959 0.0959 0.0959 0.0959 0.1417 0.1417 0.1417 0.1417 02364131 00125121 00885401 02319438 02364158 00786543 00885428 02319446 02246691 02252600 02017709 Apo-Hydromorphone 4mg tab Dilaudid 4mg tab pms-Hydromorphone 4mg tab Teva-Hydromorphone 4mg tab Apo-Hydromorphone 8mg tab Dilaudid 8mg tab pms-Hydromorphone 8mg tab Teva-Hydromorphone 8mg tab Apo-Hydroxyquine 200mg tab MYLAN-Hydroxychloroquine 200mg tab Plaquenil 200mg tab APX PFR PMS TEV APX PFR PMS TEV APX MYL SAV 0.2240 0.2240 0.2240 0.2240 0.3528 0.3528 0.3528 0.3528 0.2620 0.2620 0.2620 hydroxyurea 500mg cap 00465283 02343096 02242920 Hydrea 500mg cap Hydroxyurea 500mg cap MYLAN-Hydroxyurea 500mg cap BRI SAS MYL 1.0203 1.0203 1.0203 hydroxyzine HCl 10mg cap (exception status) 00646059 Apo-Hydroxyzine 10mg cap APX 0.1116 00738824 00646024 Novo-Hydroxyzin 10mg cap Apo-Hydroxyzine 25mg cap TEV APX 0.1116 0.1425 00738832 00646016 Novo-Hydroxyzin 25mg cap Apo-Hydroxyzine 50mg cap TEV APX 0.1425 0.2068 00738840 Novo-Hydroxyzin 50mg cap TEV 0.2068 00441651 02242632 00506052 00506052 02317338 Apo-Ibuprofen 300mg tab Motrin IB 300mg tab APC-Ibuprofen 400mg tab Apo-Ibuprofen 400mg tab Jamp-Ibuprofen 400mg tab APX JNJ APX APX JPC 0.1087 0.1087 0.0372 0.0372 0.0372 hydromorphone 20mg/mL inj hydromorphone 50mg/mL inj hydromorphone 1mg/mL oral sol hydromorphone HCl 1mg tab hydromorphone HCl 2mg tab hydromorphone HCl 4mg tab hydromorphone HCl 8mg tab hydroxychloroquine 200mg tab hydroxyzine HCl 25mg cap (exception status) hydroxyzine HCl 50mg cap (exception status) ibuprofen 300mg tab ibuprofen 400mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 37 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength ibuprofen 400mg tab DIN 02242658 00629340 Brand Motrin IB 400mg tab Novo-Profen 400mg tab MFR MRP JNJ 0.0372 TEV 0.0372 ibuprofen 600mg tab 00585114 00629359 Apo-Ibuprofen 600mg tab Novo-Profen 600mg tab APX TEV 0.1313 0.1313 idoxuridine 0.1% sol 02237187 02253275 Sandoz Idoxuridine 0.1% sol (discontinued) Gleevec 100mg tab SDZ NVR 4.9900 imatinib 400mg tab (exception status) imipramine 25mg tab 02253283 Gleevec 400mg tab NVR 00312797 Imipramine 25mg tab AAP 0.2682 imipramine 50mg tab 00326852 00611158 00337420 Imipramine 50mg tab Apo-Indomethacin 25mg cap (discontinued) Novo-Methacin 25mg cap AAP APX TEV 0.5232 0.0871 0.0871 indomethacin 50mg cap 00611166 00337439 Apo-Indomethacin 50mg cap (discontinued) Novo-Methacin 50mg cap APX TEV 0.1511 0.1511 indomethacin 50mg supp 02231799 Sandoz Indomethacin 50mg supp SDZ 0.8400 indomethacin 100mg supp 01934139 02231800 ratio-Indomethacin 100mg supp Sandoz Indomethacin 100mg supp TEV SDZ 0.8920 0.8920 infliximab 100mg IV inj (exception status) 02244016 Remicade 100mg pdr for inj SCH ipratropium bromide 200mcg/mL & salbutamol 1mg/mL unit dose inh sol (exception status) 02231675 Combivent UD inh sol BOE 0.2936 02272695 02243789 MYLAN-Combo Sterinebs UD inh sol ratio-IPRA SAL UD inh sol MYL TEV 0.2936 0.2936 02231135 pms-Ipratropium 125mcg/mL UD inh sol PMS 0.1579 02097176 ratio-Ipratropium 125mcg/mL UD inh sol TEV 0.1579 02126222 Apo-Ipravent 250mcg/mL inh sol (20mL) APX 0.3157 02239131 02210479 02231136 02216221 MYLAN-Ipratropium 250mcg/mL inh sol (20mL) Novo-Ipramide 250mcg/mL inh sol (20mL) pms-Ipratropium 250mcg/mL inh sol (20mL) MYLAN-Ipratropium 250mcg/mL UD inh sol (1mL) MYL TEV PMS MYL 0.3157 0.3157 0.3157 02231244 02097168 pms-Ipratropium 250mcg/mL UD inh sol (1mL) ratio-Ipratropium 250mcg/mL UD inh sol (1mL) PMS TEV 0.3157 0.3157 02216221 MYLAN-Ipratropium 250mcg/mL UD inh sol (2mL) MYL 0.3157 02231245 02097168 02163705 pms-Ipratropium 250mcg/mL UD inh sol (2mL) ratio-Ipratropium 250mcg/mL UD inh sol (2mL) Atrovent 0.3% nasal spray PMS TEV BOE 0.3157 0.3157 0.0508 02239627 pms-Ipratropium 0.3% nasal spray PMS 0.0508 02246084 Apo-Ipravent 0.6% nasal spray APX 0.1355 02163713 02386968 02237923 Atrovent 0.6% nasal spray Apo-Irbesartan 75mg tab Avapro 75mg tab BOE APX BRI 0.1355 0.4234 0.4234 imatinib 100mg tab (exception status) indomethacin 25mg cap ipratropium bromide 125mcg/mL unit dose inh sol (2mL) (exception status) ipratropium bromide 250mcg/mL inh sol (20mL) (exception status) ipratropium bromide 250mcg/mL unit dose inh sol (1mL) (exception status) ipratropium bromide 250mcg/mL unit dose inh sol (2mL) (exception status) ipratropium bromide 0.3% nasal spray (21mcg/dose) ipratropium bromide 0.6% nasal spray (42mcg/dose) irbesartan 75mg tab PRP 29.5926 118.3702 1050.4970 0.3157 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 38 of 87 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength irbesartan 75mg tab DIN 02328070 02372347 02347296 02317060 02316390 02328461 02315971 Brand CO Irbesartan 75mg tab Irbesartan 75mg tab MYLAN-Irbesartan 75mg tab pms-Irbesartan 75mg tab ratio-Irbesartan 75mg tab Sandoz Irbesartan 75mg Teva-Irbesartan 75mg tab MFR MRP COB 0.4234 SAS 0.4234 MYL 0.4234 PMS 0.4234 TEV 0.4234 SDZ 0.4234 TEV 0.4234 irbesartan 150mg tab 02386976 02237924 02328089 02372371 02347318 02317079 02316404 02328488 02315998 Apo-Irbesartan 150mg tab Avapro 150mg tab CO Irbesartan 150mg tab Irbesartan 150mg tab MYLAN-Irbesartan 150mg tab pms-Irbesartan 150mg tab ratio-Irbesartan 150mg tab Sandoz Irbesartan 150mg tab Teva-Irbesartan 150mg tab APX BRI COB SAS MYL PMS TEV SDZ TEV 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 irbesartan 300mg tab 02386984 02237925 02328100 02372398 02347326 02317087 02316412 02328496 02316005 Apo-Irbesartan 300mg tab Avapro 300mg tab CO Irbesartan 300mg tab Irbesartan 300mg tab MYLAN-Irbesartan 300mg tab pms-Irbesartan 300mg tab ratio-Irbesartan 300mg tab Sandoz Irbesartan 300mg tab Teva-Irbesartan 300mg tab APX BRI COB SAS MYL PMS TEV SDZ TEV 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 irbesartan 150mg & hydrochlorothiazide 12.5mg tab 02241818 Avalide 150/12.5mg tab BRI 0.4234 02357399 02372886 02328518 02363208 02330512 02337428 02316013 CO Irbesartan/HCT 150/12.5mg tab Irbesartan/HCTZ 150/12.5mg tab pms-Irbesartan-HCTZ 150/12.5 mg tab RAN-Irbesartan HCTZ 150/12.5mg tab ratio-Irbesartan HCTZ 150/12.5 mg tab Sandoz Irbesartan HCT 150/12.5mg tab Teva-Irbesartan/HCTZ 150/12.5mg tab COB SAS PMS RAN TEV SDZ TEV 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 02241819 Avalide 300/12.5mg tab BRI 0.4234 02357402 02372894 02328526 02363216 02330520 02337436 02316021 02357410 CO Irbesartan/HCT 300/12.5mg tab Irbesartan/HCTZ 300/12.5mg tab pms-Irbesartan-HCTZ 300/12.5 mg tab RAN-Irbesartan HCTZ 300/12.5mg tab ratio-Irbesartan HCTZ 300/12.5 mg tab Sandoz Irbesartan HCT 300/12.5mg tab Teva-Irbesartan/HCTZ 300/12.5mg tab CO Irbesartan/HCT 300/25mg tab COB SAS PMS RAN TEV SDZ TEV COB 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 0.4234 0.4206 02372908 Irbesartan/HCTZ 300/25mg tab SAS 0.4206 irbesartan 300mg & hydrochlorothiazide 12.5mg tab irbesartan 300mg & hydrochlorothiazide 25mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 39 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength irbesartan 300mg & hydrochlorothiazide 25mg tab DIN 02328534 Brand pms-Irbesartan-HCTZ 300/25 mg tab MFR MRP PMS 0.4206 02363224 02330539 02337444 02316048 RAN-Irbesartan HCTZ 300/25mg tab ratio-Irbesartan HCTZ 300/25 mg tab Sandoz Irbesartan HCT 300/25mg tab Teva-Irbesartan/HCTZ 300/25mg tab RAN TEV SDZ TEV 0.4206 0.4206 0.4206 0.4206 isosorbide dinitrate 5mg SL tab 00670944 ISDN 5mg tab AAP 0.0674 isosorbide dinitrate 10mg tab isosorbide dinitrate 30mg tab 00441686 ISDN 10mg tab AAP 0.0397 00441694 02272830 02126559 02301288 ISDN 30mg tab Apo-ISMN 60mg SR tab Imdur 60mg ER tab pms-ISMN 60mg SR tab AAP APX AZE PMS 0.0930 0.3523 0.3523 0.3523 00582344 02257955 00582352 02257963 Accutane 10mg cap Clarus 10mg cap Accutane 40mg cap Clarus 40mg cap HLR MYL HLR MYL 0.9313 0.9313 1.9003 1.9003 ketoconazole 2% cr ketoconazole 200mg tab 02245662 02237235 02231061 Ketoderm 2% cr Apo-Ketoconazole 200mg tab Novo-Ketoconazole 200mg tab TPH APX TEV 0.3166 0.9393 0.9393 ketoprofen 50mg cap 00790427 Apo-Keto 50mg cap AAP 0.1750 ketoprofen 50mg EC tab 00790435 Apo-Keto-E 50mg EC tab AAP 0.1750 ketoprofen 100mg EC tab 00842664 02172577 Apo-Keto-E 100mg EC tab Ketoprofen SR 200mg EC tab AAP AAP 0.3500 0.7000 isosorbide mononitrate 60mg SR tab isotretinoin 10mg cap isotretinoin 40mg cap ketoprofen 200mg SR tab ketorolac 30mg/mL inj 02239944 Ketorolac 30mg/mL inj SDZ 4.3000 ketorolac 0.5% oph sol 01968300 02245821 02247461 Acular 0.5% oph sol Apo-Ketorolac 0.5% oph sol ratio-Ketorolac 0.5% oph sol ALL APX TEV 1.6000 1.6000 1.6000 ketotifen fumarate 1mg tab 02230730 00577308 Novo-Ketotifen 1mg tab Zaditen 1mg tab TEV TEV 1.6722 1.6722 ketotifen fumarate 1mg/5mL syr 02176084 Novo-Ketotifen 1mg/5mL inj TEV 0.1330 lactulose 667mg/mL o/l (exception status) 02242814 02295881 00854409 02331551 02393239 02239193 02245208 02381354 02142082 02343010 02265494 02248232 02246897 02243352 Apo-Lactulose 667mg/mL o/l Jamp-Lactulose 667mg/mL o/l ratio-Lactulose 667mg/mL o/l Teva-Lactulose 667mg/mL o/l Apo-Lamivudine HBV 100mg Tab Heptovir 100mg Tab Apo-Lamotrigine 25mg tab Auro-Lamotrigine 25mg tab Lamictal 25mg tab Lamotrigine 25mg tab MYLAN-Lamotrigine 25mg tab Novo-Lamotrigine 25mg tab pms-Lamotrigine 25mg tab ratio-Lamotrigine 25mg tab APX JPC TEV TEV APX VIV APX ARO GSK SAS MYL TEV PMS TEV 0.0145 0.0145 0.0145 0.0145 3.5316 3.5316 0.1310 0.1310 0.1310 0.1310 0.1310 0.1310 0.1310 0.1310 lamivudine 100mg tab (exception status) lamotrigine 25mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 40 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength lamotrigine 100mg tab DIN 02245209 02381362 02142104 02343029 02265508 02248233 02246898 02243353 Brand Apo-Lamotrigine 100mg tab Auro-Lamotrigine 100mg tab Lamictal 100mg tab Lamotrigine 100mg tab MYLAN-Lamotrigine 100mg tab Novo-Lamotrigine 100mg tab pms-Lamotrigine 100mg tab ratio-Lamotrigine 100mg tab MFR MRP APX 0.5229 ARO 0.5229 GSK 0.5229 SAS 0.5229 MYL 0.5229 TEV 0.5229 PMS 0.5229 TEV 0.5229 lamotrigine 150mg tab 02245210 02381370 02142112 02343037 02265516 02248234 02246899 02246963 Apo-Lamotrigine 150mg tab Auro-Lamotrigine 150mg tab Lamictal 150mg tab Lamotrigine 150mg tab MYLAN-Lamotrigine 150mg tab Novo-Lamotrigine 150mg tab pms-Lamotrigine 150mg tab ratio-Lamotrigine 150mg tab APX ARO GSK SAS MYL TEV PMS TEV lancets 97799689 97799691 97799494 97799495 97799817 97799816 97799946 97799945 97799942 97799917 97799918 97799882 97799883 97799466 97799540 97799825 97799826 97799766 97799767 97799592 97799591 97799810 97799807 97799431 97799501 97799765 97799970 97799948 02293811 Abbott Thin (200) Abbott Thin 28g (100) Accu-Chek Fastclix Lancets (102) Accu-Chek Fastclix Lancets (204) Accu-Chek Multiclix (102) Accu-Chek Multiclix (204) Accu-Chek Softclix (100) Accu-Chek Softclix (200) Accu-Chek Softclix Pro (200) Ascensia Microlet Ascensia Microlet (100) BD Ultra-Fine 33g (100) (discontinued) BD Ultra-Fine 33g (200) BGStar Lancets (100) EZ Health (100) Finger Stix (200) FreeStyle (100) iTest 28g (100) iTest 33g (100) Medlance Plus Lite 25g (200) Medlance Plus Universal 21G (200) MPD Thin MPD Ultra Thin (100) OneTouch Delica 30G (100) OneTouch Delica 33G (100) OneTouch Sure Soft (200) OneTouch Ultra Soft (100) Safe-T-Pro (200) Apo-Lansoprazole 15mg DR cap MID MID BOM BOM BOM BOM BOM BOM BOM BDD BDD BTD BTD SAV THI BDD MID AUT AUT MPD MPD MPD MPD LFS LFS LFS LFS BOM APX lansoprazole 15mg cap (exception status) 0.7706 0.7706 0.7706 0.7706 0.7706 0.7706 0.7706 0.7706 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 41 of 87 PRP 0.0445 0.0500 0.0500 0.0500 0.0500 0.0500 0.0500 0.0500 0.0500 0.0500 0.0500 0.0500 0.0495 0.0500 0.0500 0.0500 0.0500 0.0465 0.0404 0.0500 0.0500 0.0318 0.0318 0.0500 0.0500 0.0500 0.0500 0.0500 0.3500 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 1 Generic Name and Strength lansoprazole 15mg cap (exception status) DIN 02357682 Brand Lansoprazole 15mg DR cap MFR MRP SAS 02353830 02280515 02165503 02385643 MYLAN-Lansoprazole 15mg DR cap Novo-Lansoprazole 15mg DR cap Prevacid 15mg cap Sandoz Lansoprazole 15mg DR cap MYL TEV ABB SDZ lansoprazole 30mg cap (exception status) 02293838 02357690 02353849 02280523 02165511 02385651 Apo-Lansoprazole 30mg DR cap Lansoprazole 30mg DR cap MYLAN-Lansoprazole 30mg DR cap Novo-Lansoprazole 30mg DR cap Prevacid 30mg cap Sandoz Lansoprazole 30mg DR cap APX SAS MYL TEV ABB SDZ 0.7000 0.7000 0.7000 0.7000 0.7000 0.7000 latanopost 50mcg/mL oph sol 02296527 02254786 02373041 02231493 02256495 02241888 02351668 02319225 02261251 02288265 02283964 Apo-Latanoprost 0.005% oph sol CO Latanoprost 0.005% oph sol GD-Lantanoprost 0.005% oph sol Xalatan 0.005% oph sol Apo-Leflunomide 10mg tab Arava 10mg tab Leflunomide 10mg tab MYLAN-Leflunomide 10mg tab Novo-Leflunomide 10mg tab pms-Leflunomide 10mg tab Sandoz Leflunomide 10mg tab APX COB GMD PFI APX SAV SAS MYL TEV PMS SDZ 3.8542 3.8542 3.8542 3.8542 3.7597 3.7597 3.7597 3.7597 3.7597 3.7597 3.7597 02256509 02241889 02351676 02319233 02261278 02288273 02283972 02358514 02231384 02373009 02338459 02348969 02347997 02373424 02322315 02372169 02309114 02372282 02344815 02285924 Apo-Leflunomide 20mg tab Arava 20mg tab Leflunomide 20mg tab MYLAN-Leflunomide 20mg tab Novo-Leflunomide 20mg tab pms-Leflunomide 20mg tab Sandoz Leflunomide 20mg tab Apo-Letrozole 2.5mg tab Femara 2.5mg tab Jamp-Letrozole 2.5mg tab Letrozole 2.5mg tab (AHI) Letrozole 2.5mg tab (COB) Letrozole 2.5mg tab (TEV) Mar-Letrozole 2.5mg tab MED-Letrozole 2.5mg tab Myl-Letrozole 2.5mg tab pms-Letrozole 2.5mg tab RAN-Letrozole 2.5mg tab Sandoz Letrozole 2.5mg tab Apo-Levetiracetam 250mg tab APX SAV SAS MYL TEV PMS SDZ APX NVR JPC AHI COB TEV MAR GMP MYL PMS RAN SDZ APX 3.7597 3.7597 3.7597 3.7597 3.7597 3.7597 3.7597 2.0662 2.0662 2.0662 2.0662 2.0662 2.0662 2.0662 2.0662 2.0662 2.0662 2.0662 2.0662 0.8000 02375249 Auro-Levetiracetam 250mg tab ARO 0.8000 leflunomide 10mg tab (exception status) leflunomide 20mg tab (exception status) letrozole 2.5 tab levetiracetam 250mg tab (exception status) 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Page 42 of 87 PRP 0.3500 0.3500 0.3500 0.3500 0.3500 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. Version: NS Pharmacare Reimbursement List Effective April 2013 2 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength levetiracetam 250mg tab (exception status) DIN 02274183 Brand CO Levetiracetam 250mg tab MFR MRP COB 0.8000 02247027 02353342 02296101 Keppra 250mg tab Levetiracetam 250mg tab pms-Levetiracetam 250mg tab UCB SAS PMS 0.8000 0.8000 0.8000 02285932 Apo-Levetiracetam 500mg tab APX 0.9750 02375257 02274191 02247028 02353350 02296128 02285940 Auro-Levetiracetam 500mg tab CO Levetiracetam 500mg tab Keppra 500mg tab Levetiracetam 500mg tab pms-Levetiracetam 500mg tab Apo-Levetiracetam 750mg tab ARO COB UCB SAS PMS APX 0.9750 0.9750 0.9750 0.9750 0.9750 1.3500 02375265 02274205 02247029 02353369 02296136 Auro-Levetiracetam 750mg tab CO Levetiracetam 750mg tab Keppra 750mg tab Levetiracetam 750mg tab pms-Levetiracetam 750mg tab ARO COB UCB SAS PMS 1.3500 1.3500 1.3500 1.3500 1.3500 02031159 02241715 00637661 02237991 02031167 02241716 ratio-Levobunolol 0.25% oph sol Sandoz Levobunolol 0.25% oph sol (discontinued) Betagan 0.5% oph sol pms-Levobunolol 0.5% oph sol ratio-Levobunolol 0.5% oph sol Sandoz Levobunolol 0.5% oph sol TEV SDZ ALL PMS TEV SDZ 1.9143 1.9143 1.1515 1.1515 1.1515 1.1515 02195933 02244494 00355658 02195941 02244495 00513997 Apo-Levocarb 100/10mg tab Novo-Levocarbidopa 100/10mg tab Sinemet 100/10mg tab Apo-Levocarb 100/25mg tab Novo-Levocarbidopa 100/25mg tab Sinemet 100/25mg tab APX TEV FRS APX TEV FRS 0.1877 0.1877 0.1877 0.2803 0.2803 0.2803 levodopa 250mg & carbidopa 25mg tab 02195968 02244496 00328219 Apo-Levocarb 250/25mg tab Novo-Levocarbidopa 250/25mg tab Sinemet 250/25mg tab APX TEV FRS 0.3129 0.3129 0.3129 levodopa 100mg & carbidopa 25mg cr tab 02272873 02028786 02245211 00870935 02284707 02315424 02236841 02313979 02248262 02284677 02298635 Apo-Levocarb CR 100/25mg tab Sinemet CR 100/25mg tab Levocarb CR 200/50mg tab Sinemet CR 200/50mg tab Apo-Levofloxacin 250mg tab CO Levofloxacin 250mg tab Levaquin 250mg tab MYLAN-Levofloxacin 250mg tab Novo-Levofloxacin 250mg tab pms-Levofloxacin 250mg tab Sandoz Levofloxacin 250mg tab AAP FRS AAP FRS APX COB JAN MYL TEV PMS SDZ 0.5562 0.5562 1.0850 1.0850 1.8538 1.8538 1.8538 1.8538 1.8538 1.8538 1.8538 levetiracetam 500mg tab (exception status) levetiracetam 750mg tab (exception status) levobunolol HCl 0.25% oph sol levobunolol HCl 0.5% oph sol levodopa 100mg & carbidopa 10mg tab levodopa 100mg & carbidopa 25mg tab levodopa 200mg & carbidopa 50mg cr tab levofloxacin 250mg tab (exception status) Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 43 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength levofloxacin 500mg tab (exception status) DIN 02284715 02315432 02236842 02313987 02248263 02284685 02298643 Brand Apo-Levofloxacin 500mg tab CO Levofloxacin 500mg tab Levaquin 500mg tab MYLAN-Levofloxacin 500mg tab Novo-Levofloxacin 500mg tab pms-Levofloxacin 500mg tab Sandoz Levofloxacin 500mg tab MFR MRP APX 2.1125 COB 2.1125 JAN 2.1125 MYL 2.1125 TEV 2.1125 PMS 2.1125 SDZ 2.1125 levonorgestrel 0.10mg & ethinyl estradiol 0.02mg tab (21) 02236974 Alesse 21 Day WAY 0.4636 02387875 02298538 02388138 Alysena 21 Day Aviane 21 Day (discontinued) ESME 21 Day APX APX MYL 0.4636 0.4636 0.4636 02236975 Alesse 28 Day WAY 0.3477 02387883 02298546 02388146 02042320 Alysena 28 Day Aviane 28 Day (discontinued) ESME 28 Day Min-Ovral 21 Day APX APX MYL WAY 0.3477 0.3477 0.3477 0.4636 02295946 Portia 21 Day APX 0.4636 02042339 Min-Ovral 28 Day WAY 0.3477 levonorgestrel 0.10mg & ethinyl estradiol 0.02mg tab (28) levonorgestrel 0.15mg & ethinyl estradiol 0.03mg tab (21) levonorgestrel 0.15mg & ethinyl estradiol 0.03mg tab (28) 02295954 Portia 28 Day APX 0.3477 lidocaine 5% oint 02083795 00001961 Lidodan 5% oint Xylocaine 5% oint ODN AZE 0.3967 0.3967 linezolid 600mg tab 02243684 Zyvoxam 600mg tab PFI lisinopril 5mg tab 02217481 02271443 02361531 02274833 02285061 02285118 02292203 00839388 02294230 02256797 02299879 02289199 02049333 02217503 02271451 02361558 02274841 02285126 02292211 00839396 Apo-Lisinopril 5mg tab CO Lisinopril 5mg tab Jamp-Lisinopril 5mg tab MYLAN-Lisinopril 5mg tab Novo-Lisinopril (Type P) 5mg tab Novo-Lisinopril (Type Z) 5mg tab pms-Lisinopril 5mg tab Prinivil 5mg tab RAN-Lisinopril 5mg tab ratio-Lisinopril P 5mg tab ratio-Lisinopril Z 5mg tab Sandoz Lisinopril 5mg tab Zestril 5mg tab Apo-Lisinopril 10mg tab CO Lisinopril 10mg tab Jamp-Lisinopril 10mg tab MYLAN-Lisinopril 10mg tab Novo-Lisinopril Z 10mg tab pms-Lisinopril 10mg tab Prinivil 10mg tab APX COB JPC MYL TEV TEV PMS FRS RAN TEV TEV SDZ AZE APX COB JPC MYL TEV PMS FRS lisinopril 10mg tab 78.2560 0.2100 0.2100 0.2100 0.2100 0.2100 0.2100 0.2100 0.2100 0.2100 0.2100 0.2100 0.2100 0.2100 0.2522 0.2522 0.2522 0.2522 0.2522 0.2522 0.2522 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 44 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength lisinopril 10mg tab DIN 02294249 02256800 02299887 02289202 02285088 02049376 Brand RAN-Lisinopril 10mg tab ratio-Lisinopril P 10mg tab ratio-Lisinopril Z 10mg tab Sandoz Lisinopril 10mg tab Teva-Lisinopril (Type P) 10mg tab Zestril 10mg tab MFR MRP RAN 0.2522 TEV 0.2522 TEV 0.2522 SDZ 0.2522 TEV 0.2522 AZE 0.2522 lisinopril 20mg tab 02217511 02271478 02361566 02274868 02285134 02292238 00839418 02294257 02256819 02299895 02289229 02285096 02049384 Apo-Lisinopril 20mg tab CO Lisinopril 20mg tab Jamp-Lisinopril 20mg tab MYLAN-Lisinopril 20mg tab Novo-Lisinopril Z 20mg tab pms-Lisinopril 20mg tab Prinivil 20mg tab RAN-Lisinopril 20mg tab ratio-Lisinopril P 20mg tab (discontinued) ratio-Lisinopril Z 20mg tab Sandoz Lisinopril 20mg tab Teva-Lisinopril (Type P) 20mg tab Zestril 20mg tab APX COB JPC MYL TEV PMS FRS RAN TEV TEV SDZ TEV AZE 0.3032 0.3032 0.3032 0.3032 0.3032 0.3032 0.3032 0.3032 0.3032 0.3032 0.3032 0.3032 0.3032 lisinopril 10mg & hydrochlorothiazide 12.5mg tab 02261979 Apo-Lisinopril/HCTZ 10/12.5mg tab APX 0.3001 02362945 02297736 02302136 02301768 02302365 02103729 Lisinopril/HCTZ 10/12.5mg (Type Z) tab MYLAN-Lisinopril/HCTZ 10/12.5mg tab Novo-Lisinopril/HCTZ (Type P) 10/12.5mg tab Novo-Lisinopril/HCTZ (Type Z) 10/12.5mg tab Sandoz Lisinopril HCT 10/12.5mg tab Zestoretic 10/12.5mg tab SAS MYL TEV TEV SDZ AZE 0.3001 0.3001 0.3001 0.3001 0.3001 0.3001 02261987 Apo-Lisinopril/HCTZ 20/12.5mg tab APX 0.3605 02362953 02297744 02302144 00884413 02302373 02301776 02045737 02261995 Lisinopril/HCTZ 20/12.5mg (Type Z) tab MYLAN-Lisinopril/HCTZ 20/12.5mg tab Novo-Lisinopril/HCTZ (Type P) 20/12.5mg tab Prinzide 20/12.5mg tab Sandoz Lisinopril HCT 20/12.5mg tab Teva-Lisinopril/HCTZ (Type Z) 20/12.5mg tab Zestoretic 20/12.5mg tab Apo-Lisinopril/HCTZ 20/25mg tab SAS MYL TEV FRS SDZ TEV AZE APX 0.3605 0.3605 0.3605 0.3605 0.3605 0.3605 0.3605 0.3605 02362961 02297752 02302152 02301784 02302381 02045729 Lisinopril/HCTZ 20/25mg (Type Z) tab MYLAN-Lisinopril/HCTZ 20/25mg tab Novo-Lisinopril/HCTZ (Type P) 20/25mg tab Novo-Lisinopril/HCTZ (Type Z) 20/25mg tab Sandoz Lisinopril HCT 20/25mg tab Zestoretic 20/25mg tab SAS MYL TEV TEV SDZ AZE 0.3605 0.3605 0.3605 0.3605 0.3605 0.3605 02266695 02242837 Lithmax SR 300mg tab Apo-Lithium Carbonate 150mg cap AAP APX 0.2708 0.0422 lisinopril 20mg & hydrochlorothiazide 12.5mg tab lisinopril 20mg & hydrochlorothiazide 25mg tab lithium 300mg SR tab lithium 150mg cap (Carbolith) Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 45 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength lithium 150mg cap (Carbolith) DIN 00461733 02216132 Brand Carbolith 150mg cap pms-Lithium Carbonate 150mg cap MFR MRP VLN 0.0422 PMS 0.0422 lithium 300mg cap (Carbolith) 02242838 00236683 02216140 Apo-Lithium Carbonate 300mg cap Carbolith 300mg cap pms-Lithium Carbonate 300mg cap APX VLN PMS 0.0443 0.0443 0.0443 lithium 600mg cap 02011239 02216159 Carbolith 600mg cap pms-Lithium Carbonate 600mg cap VLN PMS 0.1530 0.1530 lithium 150mg cap (Lithane) 02242837 02013231 02242838 00406775 Apo-Lithium Carbonate 150mg cap Lithane 150mg cap Apo-Lithium Carbonate 300mg cap Lithane 300mg cap APX ERF APX ERF 0.0422 0.0422 0.0443 0.0443 loperamide 2mg caplet 02212005 02183862 02132591 02228351 02257564 Apo-Loperamide 2mg caplet Imodium 2mg caplet Novo-Loperamide 2mg caplet pms-Loperamide 2mg caplet Sandoz Loperamide 2mg caplet APX JNJ TEV PMS SDZ 0.1255 0.1255 0.1255 0.1255 0.1255 loperamide HCl 0.2mg/mL o/l 02016095 02243880 02243880 00782696 00655740 02041413 02351072 00711101 00728187 00655759 02041421 02351080 00637742 00728195 00655767 02041448 02351099 00637750 00728209 02243278 02379058 02354829 02182815 02388863 02368277 02309750 02313332 02380838 pms-Loperamide 0.2mg/mL o/l APC-Loratadine 10mg tab Apo-Loratadine 10mg tab Claritin 10mg tab Apo-Lorazepam 0.5mg tab Ativan 0.5mg tab Lorazepam 0.5mg tab Novo-Lorazem 0.5mg tab pms-Lorazepam 0.5mg tab Apo-Lorazepam 1mg tab Ativan 1mg tab Lorazepam 1mg tab Novo-Lorazem 1mg tab pms-Lorazepam 1mg tab Apo-Lorazepam 2mg tab Ativan 2mg tab Lorazepam 2mg tab Novo-Lorazem 2mg tab pms-Lorazepam 2mg tab Lorazepam 4mg/mL inj Apo-Losartan 25mg tab CO Losartan 25mg tab Cozaar 25mg tab Losartan 25mg tab MYLAN-Losartan 25mg tab pms-Losartan 25mg tab Sandoz Losartan 25mg tab Teva-Losartan 25mg tab PMS APX APX SCH APX WAY SAS TEV PMS APX WAY SAS TEV PMS APX WAY SAS TEV PMS SDZ APX COB FRS SAS MYL PMS SDZ TEV 0.1050 0.6267 0.6267 0.6267 0.0359 0.0359 0.0359 0.0359 0.0359 0.0447 0.0447 0.0447 0.0447 0.0447 0.0699 0.0699 0.0699 0.0699 0.0699 2.9900 0.4407 0.4407 0.4407 0.4407 0.4407 0.4407 0.4407 0.4407 lithium 300mg cap (Lithane) loratadine 10mg tab (exception status) lorazepam 0.5mg tab lorazepam 1mg tab lorazepam 2mg tab lorazepam 4mg/mL inj losartan 25mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 46 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength losartan 50mg tab DIN 02353504 02354837 02182874 02388871 02368285 02309769 02313340 02357968 Brand Apo-Losartan 50mg tab CO Losartan 50mg tab Cozaar 50mg tab Losartan 50mg tab MYLAN-Losartan 50mg tab pms-Losartan 50mg tab Sandoz Losartan 50mg tab Teva-Losartan 50mg tab MFR MRP APZ 0.4407 COB 0.4407 FRS 0.4407 SAS 0.4407 MYL 0.4407 PMS 0.4407 SDZ 0.4407 TEV 0.4407 losartan 100mg tab 02353512 02354845 02182882 02388898 02368293 02309777 02313359 02357976 Apo-Losartan 100mg tab CO Losartan 100mg tab Cozaar 100mg tab Losartan 100mg tab MYLAN-Losartan 100mg tab pms-Losartan 100mg tab Sandoz Losartan 100mg tab Teva-Losartan 100mg tab APX COB FRS SAS MYL PMS SDZ TEV 0.4407 0.4407 0.4407 0.4407 0.4407 0.4407 0.4407 0.4407 losartan 50mg & hydrochlorothiazide 12.5mg tab 02371235 Apo-Losartan/HCTZ 50/12.5mg tab APX 0.4407 02388251 02230047 02378078 02392224 02313375 02358263 02371243 CO Losartan/HCT 50/12.5mg tab Hyzaar 50/12.5mg tab MYLAN-Losartan HCTZ 50/12.5mg tab pms-Losartan-HCTZ 50/12.5mg tab Sandoz Losartan HCT 50/12.5mg tab Teva-Losartan/HCTZ 50/12.5mg tab Apo-Losartan HCTZ 100/12.5mg tab COB FRS MYL PMS SDZ TEV APX 0.4407 0.4407 0.4407 0.4407 0.4407 0.4407 0.4314 02388278 02297841 02378086 02392232 02362449 02377144 CO Losartan/HCT 100/12.5mg tab Hyzaar 100/12.5mg tab MYLAN-Losartan HCTZ 100/12.5mg tab pms-Losartan-HCTZ 100/12.5mg tab Sandoz Losartan HCT 100/12.5mg tab Teva-Losartan/HCTZ 100/12.5mg tab COB FRS MYL PMS SDZ TEV 0.4314 0.4314 0.4314 0.4314 0.4314 0.4314 02371251 Apo-Losartan HCTZ 100/25mg tab APX 0.4407 02388286 02241007 02378094 02392240 02313383 02377152 CO Losartan/HCT 100/25mg tab Hyzaar DS 100/25mg tab MYLAN-Losartan HCTZ 100/25mg tab pms-Losartan-HCTZ 100/25mg tab Sandoz Losartan HCT DS 100/25mg tab Teva-Losartan/HCTZ 100/25mg tab COB FRS MYL PMS SDZ TEV 0.4407 0.4407 0.4407 0.4407 0.4407 0.4407 02220172 02248572 02353229 00795860 02243127 02246542 Apo-Lovastatin 20mg tab CO Lovastatin 20mg tab Lovastatin 20mg tab Mevacor 20mg tab MYLAN-Lovastatin 20mg tab Novo-Lovastatin 20mg tab APX COB SAS FRS MYL TEV 0.7231 0.7231 0.7231 0.7231 0.7231 0.7231 losartan 100mg & hydrochlorothiazide 12.5mg tab losartan 100mg & hydrochlorothiazide 25mg tab lovastatin 20mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 47 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength lovastatin 20mg tab DIN 02246013 02245822 02247056 Brand pms-Lovastatin 20mg tab ratio-Lovastatin 20mg tab Sandoz Lovastatin 20mg tab MFR MRP PMS 0.7231 TEV 0.7231 SDZ 0.7231 lovastatin 40mg tab 02220180 02248573 02353237 00795852 02243129 02246543 02246014 02245823 02247057 02230837 Apo-Lovastatin 40mg tab CO Lovastatin 40mg tab Lovastatin 40mg tab Mevacor 40mg tab MYLAN-Lovastatin 40mg tab Novo-Lovastatin 40mg tab pms-Lovastatin 40mg tab ratio-Lovastatin 40mg tab Sandoz Lovastatin 40mg tab Xylac 5mg tab APX COB SAS FRS MYL TEV PMS TEV SDZ PMS 1.3208 1.3208 1.3208 1.3208 1.3208 1.3208 1.3208 1.3208 1.3208 0.1790 02230838 02230839 Xylac 10mg tab Xylac 25mg tab PMS PMS 0.2979 0.4617 02230840 Xylac 50mg tab PMS 0.6155 02158612 02158620 02158639 02244726 02221284 00708917 Novo-Maprotiline 25mg tab Novo-Maprotiline 50mg tab Novo-Maprotiline 75mg tab Apo-Medroxy 2.5mg tab Novo-Medrone 2.5mg tab Provera 2.5mg tab TEV TEV TEV APX TEV PFI 0.5687 1.0769 1.4707 0.0642 0.0642 0.0642 medroxyprogesterone acetate 5mg tab 02244727 02221292 00030937 Apo-Medroxy 5mg tab Novo-Medrone 5mg tab Provera 5mg tab APX TEV PFI 0.1270 0.1270 0.1270 medroxyprogesterone acetate 10mg tab 02277298 02221306 00729973 02267640 00585092 Apo-Medroxy 10mg tab Novo-Medrone 10mg tab Provera 10mg tab Apo-Medroxy 100mg tab Depo-Provera 150mg/mL inj APX TEV PFI APX PFI 0.2577 0.2577 0.2577 1.2057 22.0000 02322250 Medroxyprogesterone Acetate 150mg/mL inj SDZ 22.0000 02229452 02195917 02195925 02248973 02250012 02353148 02242785 02255987 02258315 02248607 02248267 02248974 Apo-Mefenamic 250mg cap Megestrol 40mg tab Megestrol 160mg tab Apo-Meloxicam 7.5mg tab CO Meloxicam 7.5mg tab Meloxicam 7.5mg tab Mobicox 7.5mg tab MYLAN-Meloxicam 7.5mg tab Novo-Meloxicam 7.5mg tab phl-Meloxicam 7.5mg tab pms-Meloxicam 7.5mg tab Apo-Meloxicam 15mg tab AAP AAP AAP APX COB SAS BOE MYL TEV PHL PMS APX 0.5412 1.0929 4.6254 0.2804 0.2804 0.2804 0.2804 0.2804 0.2804 0.2804 0.2804 0.3235 loxapine 5mg tab loxapine 10mg tab loxapine 25mg tab loxapine 50mg tab maprotiline 25mg tab maprotiline 50mg tab maprotiline 75mg tab medroxyprogesterone acetate 2.5mg tab medroxyprogesterone acetate 100mg tab medroxyprogesterone acetate 150mg/mL inj mefenamic acid 250mg cap megestrol 40mg tab megestrol 160mg tab meloxicam 7.5mg tab meloxicam 15mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 48 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength meloxicam 15mg tab DIN 02250020 02353156 02242786 02255995 02248608 02248268 02258323 Brand CO Meloxicam 15mg tab Meloxicam 15mg tab Mobicox 15mg tab MYLAN-Meloxicam 15mg tab phl-Meloxicam 15mg tab pms-Meloxicam 15mg tab Teva-Meloxicam 15mg tab MFR MRP COB 0.3235 SAS 0.3235 BOE 0.3235 MYL 0.3235 PHL 0.3235 PMS 0.3235 TEV 0.3235 metformin HCl 500mg tab 02167786 02257726 02099233 02380196 02380722 02378620 02378841 02242794 02353377 02148765 02045710 02223562 02269031 02242974 02246820 02379767 Apo-Metformin 500mg tab CO Metformin 500mg tab Glucophage 500mg tab Jamp-Metformin 500mg tab Jamp-Metformin Blackberry 500mg tab Mar-Metformin 500mg tab Metformin 500mg tab (MAR) Metformin 500mg tab (MEL) Metformin 500mg tab (SAS) MYLAN-Metformin 500mg tab Novo-Metformin 500mg tab pms-Metformin 500mg tab RAN-Metformin 500mg tab ratio-Metformin 500mg tab Sandoz Metformin FC 500mg tab Septa-Metformin 500mg tab APX COB SAV JPC JPC MAR MAR MEL SAS MYL TEV PMS RAN TEV SDZ SPT 0.0834 0.0834 0.0834 0.0834 0.0834 0.0834 0.0834 0.0834 0.0834 0.0834 0.0834 0.0834 0.0834 0.0834 0.0834 0.0834 metformin HCl 850mg tab 02229785 02257734 02162849 02380218 02380730 02378639 02378868 02353385 02229656 02230475 02242589 02269058 02242931 02246821 02379775 02182963 02170698 02244798 02238403 02238404 Apo-Metformin 850mg tab CO Metformin 850mg tab Glucophage 850mg tab Jamp-Metformin 850mg tab Jamp-Metformin Blackberry 850mg tab Mar-Metformin 850mg tab Metformin 850mg tab (MAR) Metformin 850mg tab (SAS) MYLAN-Metformin 850mg tab Novo-Metformin 850mg tab pms-Metformin 850mg tab RAN-Metformin 850mg tab ratio-Metformin 850mg tab Sandoz Metformin FC 850mg tab Septa-Metformin 850mg tab Apo-Methotrexate 2.5mg tab Methotrexate 2.5mg tab ratio-Methotrexate Sodium 2.5mg tab Apo-Methoprazine 2mg tab Apo-Methoprazine 5mg tab APX COB SAV JPC JPC MAR MAR SAS MYL TEV PMS RAN TEV SDZ SPT APX WAY TEV APX APX 0.1205 0.1205 0.1205 0.1205 0.1205 0.1205 0.1205 0.1205 0.1205 0.1205 0.1205 0.1205 0.1205 0.1205 0.1205 0.6325 0.6325 0.6325 0.0685 0.0991 02238405 02238406 Apo-Methoprazine 25mg tab Apo-Methoprazine 50mg tab APX APX 0.2547 0.3857 methotrexate 2.5mg tab methotrimeprazine 2mg tab methotrimeprazine 5mg tab methotrimeprazine 25mg tab methotrimeprazine 50mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 49 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength methyldopa 125mg tab DIN 00360252 Brand Methyldopa 125mg tab MFR MRP AAP 0.1074 methyldopa 250mg tab 00360260 Methyldopa 250mg tab AAP 0.1555 methyldopa 500mg tab methylphenidate 10mg tab 00426830 02249324 00584991 00005606 Methyldopa 500mg tab Apo-Methylphenidate 10mg tab pms-Methylphenidate 10mg tab Ritalin 10mg tab AAP APX PMS NVR 0.2753 0.1271 0.1271 0.1271 methylphenidate 20mg tab 02249332 00585009 00005614 02247732 02315068 Apo-Methylphenidate 20mg tab pms-Methylphenidate 20mg tab Ritalin 20mg tab Concerta 18mg tab Novo-Methylphenidate ER-C 18mg tab APX PMS NVR JAN TEV 0.2359 0.2359 0.2359 1.4276 1.4276 02250241 02315076 02247733 02315084 Concerta 27mg tab Novo-Methylphenidate ER-C 27mg tab Concerta 36mg tab Novo-Methylphenidate ER-C 36mg tab JAN TEV JAN TEV 1.6475 1.6475 1.8674 1.8674 methylphenidate 54mg ER tab 02247734 02315092 Concerta 54mg tab Novo-Methylphenidate ER-C 54mg tab JAN TEV 2.3072 2.3072 methylphenidate 20mg SR tab 02266687 00632775 02320312 Apo-Methylphenidate 20mg SR tab Ritalin 20mg SR tab Sandoz Methylphenidate 20mg SR tab APX NVR SDZ 0.2820 0.2820 0.2820 methylprednisolone acetate 40mg/vial inj 01934333 02245407 Depo-Medrol 40mg/mL inj Methylprednisolone Acetate 40mg/mL inj PFI SDZ 4.5150 4.5150 methylprednisolone acetate 80mg/vial inj 01934341 02245408 00030759 Depo-Medrol 80mg/mL inj Methylprednisolone Acetate 80mg/mL inj Depo-Medrol 40mg/mL inj (PF) PFI SDZ PFI 6.9900 6.9900 4.7250 02245400 Methylprednisolone Acetate 40mg/mL inj (PF) SDZ 4.7250 00030767 Depo-Medrol 80mg/mL inj (PF) PFI 9.0300 02245406 Methylprednisolone Acetate 80mg/mL inj (PF) SDZ 9.0300 02231893 Methylprednisolone Sod. Succ. 40mg/vial inj TEV 4.2966 02231894 Methylprednisolone Sod. Succ. 125mg/vial inj TEV 9.3500 02231895 Methylprednisolone Sod Succ 500mg/vial inj TEV 22.2002 00030678 Solu-Medrol 500mg/vial inj PFI 22.2002 02241229 Methylprednisolone Sod Succ 1g/vial inj TEV 31.0000 00036137 00842826 02230431 Solu-Medrol 1g/vial inj Apo-Metoclop 5mg tab (discontinued) pms-Metoclopramide 5mg tab PFI 31.0000 APX 0.0556 PMS 0.0556 00842834 02230432 02230433 Apo-Metoclop 10mg tab (discontinued) pms-Metoclopramide 10mg tab pms-Metoclopramide 1mg/mL liq APX PMS PMS 0.0583 0.0583 0.0486 00618632 Apo-Metoprolol 50mg tab APX 0.0940 methylphenidate 18mg ER tab methylphenidate 27mg ER tab methylphenidate 36mg ER tab methylprednisolone acetate 40mg/vial inj (pf) methylprednisolone acetate 80mg/vial inj (pf) methylprednisolone sodium succinate 40mg/vial inj methylprednisolone sodium succinate 125mg/vial inj methylprednisolone sodium succinate 500mg/vial inj methylprednisolone sodium succinate 1g/vial inj metoclopramide HCl 5mg tab metoclopramide HCl 10mg tab metoclopramide HCl 1mg o/l metoprolol tartrate 50mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 50 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength metoprolol tartrate 50mg tab DIN 00749354 02356821 00397423 02350394 02174545 02230803 02354187 02247875 00648035 Brand MFR MRP Apo-Metoprolol-L 50mg tab APX 0.0940 Jamp-Metoprolol-L 50mg tab JPC 0.0940 Lopresor 50mg tab NVR 0.0940 Metoprolol Film-Coated 50mg tab SAS 0.0940 MYLAN-Metoprolol (Type L) 50mg tab MYL 0.0940 pms-Metoprolol-L 50mg tab PMS 0.0940 Sandoz Metoprolol (Type L) 50mg tab SDZ 0.0940 Sandoz Metoprolol (Type L) 50mg tab (discontinued)SDZ 0.0940 Teva-Metoprolol 50mg tab TEV 0.0940 metoprolol tartrate 100mg tab 00618640 00751170 02356848 00397431 02350408 02174553 02230804 02354195 00648043 Apo-Metoprolol 100mg tab Apo-Metoprolol-L 100mg tab Jamp-Metoprolol-L 100mg tab Lopresor 100mg tab Metoprolol Film-Coated 100mg tab MYLAN-Metoprolol (Type L) 100mg tab pms-Metoprolol-L 100mg tab Sandoz Metoprolol (Type L) 100mg tab Teva-Metoprolol 100mg tab APX APX JPC NVR SAS MYL PMS SDZ TEV 0.2050 0.2050 0.2050 0.2050 0.2050 0.2050 0.2050 0.2050 0.2050 metoprolol tartrate 100mg SR tab 02285169 00658855 02303396 02285177 00534560 02303418 Apo-Metoprolol 100mg SR tab Lopresor 100mg SR tab Sandoz Metoprolol 100mg SR tab Apo-Metoprolol 200mg SR tab Lopresor 200mg SR tab Sandoz Metoprolol 200mg SR tab APX NVR SDZ APX NVR SDZ 0.1248 0.1248 0.1248 0.2499 0.2499 0.2499 metronidazole 250mg tab mexiletine 100mg cap 00545066 Metronidazole 250mg tab AAP 0.0749 02230359 Novo-Mexiletine 100mg cap TEV 1.0203 mexiletine 200mg cap miconazole 2% vag cr 02230360 02231106 02084309 02240285 02240286 Novo-Mexiletine 200mg cap Micozole 2% vag cr Monistat 7 2% vag cr Midazolam 1mg/mL inj Midazolam 5mg/mL inj TEV TAR JNJ SDZ SDZ 1.3663 0.1511 0.1511 0.7800 4.1000 02278677 Midodrine 2.5mg tab AAP 0.3665 02278685 Midodrine 5mg tab AAP 0.6109 02084090 02287226 02230735 02108143 02294419 02237313 02084104 02287234 02230736 02108151 02294427 Apo-Minocycline 50mg cap Minocycline 50mg cap MYLAN-Minocycline 50mg cap Novo-Minocycline 50mg cap pms-Minocycline 50mg cap Sandoz Minocycline 50mg cap Apo-Minocycline 100mg cap Minocycline 100mg cap MYLAN-Minocycline 100mg cap Novo-Minocycline 100mg cap pms-Minocycline 100mg cap APX SAS MYL TEV PMS SDZ APX SAS MYL TEV PMS 0.3064 0.3064 0.3064 0.3064 0.3064 0.3064 0.5912 0.5912 0.5912 0.5912 0.5912 metoprolol tartrate 200mg SR tab midazolam 1mg/mL inj midazolam 5mg/mL inj midodrine 2.5mg tab midodrine 5mg tab minocycline HCl 50mg cap minocycline HCl 100mg cap Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 51 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength minocycline HCl 100mg cap DIN 02237314 Brand Sandoz Minocycline 100mg cap MFR MRP SDZ 0.5912 mirtazapine 30mg tab 02286629 02370689 02256118 02259354 02252279 02248762 02243910 02250608 02325187 02299801 02352826 02279894 02248542 Apo-Mirtazapine 30mg tab Mirtazapine 30mg tab MYLAN-Mirtazapine 30mg tab Novo-Mirtazapine 30mg tab phl-Mirtazapine 30mg tab pms-Mirtazapine 30mg tab Remeron 30mg tab Sandoz Mirtazapine 30mg tab Zym-Mirtazapine 30mg tab Auro-Mirtazapine OD 15mg tab GD-Mirtazapine OD 15mg tab Novo-Mirtazapine 15mg OD tab Remeron 15mg RD tab APX SAS MYL TEV PHL PMS ORG SDZ ZYM ARO GMD TEV ORG 0.4557 0.4557 0.4557 0.4557 0.4557 0.4557 0.4557 0.4557 0.4557 0.1406 0.1406 0.1406 0.1406 mirtazapine 30mg RD tab 02299828 02352834 02279908 02248543 Auro-Mirtazapine OD 30mg tab GD-Mirtazapine OD 30mg tab Novo-Mirtazapine 30mg OD tab Remeron 30mg RD tab ARO GMD TEV ORG 0.2812 0.2812 0.2812 0.2812 mirtazapine 45mg RD tab 02299836 02352842 02279916 02248544 Auro-Mirtazapine OD 45mg tab GD-Mirtazapine OD 45mg tab Novo-Mirtazapine 45mg OD tab Remeron 45mg RD tab ARO GMD TEV ORG 0.4218 0.4218 0.4218 0.4218 misoprostol 100mcg tab 02244022 02244023 Misoprostol 100mcg tab Misoprostol 200mcg tab AAP AAP 0.2804 0.4669 02232148 02239746 02232150 00899356 02239747 Apo-Moclobemide 100mg tab Novo-Moclobemide 100mg tab Apo-Moclobemide 150mg tab Manerix 150mg tab Novo-Moclobemide 150mg tab APX TEV APX MVL TEV 0.2520 0.2520 0.2120 0.2120 0.2120 02240456 02166747 02239748 00851744 02367157 00871095 02266385 00851736 02248130 02264749 02358611 Apo-Moclobemide 300mg tab Manerix 300mg tab Novo-Moclobemide 300mg tab Elocom 0.1% cr Taro-Mometasone 0.1% cr Elocom 0.1% lot Taro-Mometasone 0.1% lot Elocom 0.1% oint ratio-Mometasone 0.1% oint Taro-Mometasone 0.1% oint Sandoz Montelukast 4mg granules APX MVL TEV SCH TAR SCH TAR SCH TEV TAR SDZ 0.4164 0.4164 0.4164 0.5262 0.5262 0.3124 0.3124 0.2701 0.2701 0.2701 0.1276 02247997 02377608 Singulair 4mg/pkt granules Apo-Montelukast 4mg chewtab FRS APX 0.1276 0.5104 02379317 Montelukast 4mg chewtab SAS 0.5104 mirtazapine 15mg RD tab misoprostol 200mcg tab moclobemide 100mg tab moclobemide 150mg tab moclobemide 300mg tab mometasone 0.1% cr mometasone 0.1% lot mometasone 0.1% oint montelukast 4mg granules (exception status) montelukast 4mg chewtab (exception status) Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 52 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength montelukast 4mg chewtab (exception status) montelukast 5mg chewtab (exception status) montelukast 10mg tab (exception status) morphine sulfate 15mg SR tab morphine sulfate 30mg SR tab morphine sulfate 60mg SR tab morphine sulfate 100mg SR tab morphine sulfate 200mg SR tab morphine sulfate 10mg/mL inj morphine sulfate 15mg/mL inj mupirocin 2% oint DIN 02380749 Brand MYLAN-Montelukast 4mg chewtab MFR MRP MYL 0.5104 02354977 02330385 02243602 02355507 pms-Montelukast 4mg chewtab Sandoz Montelukast 4mg chewtab Singulair 4mg chewtab Teva-Montelukast 4mg chewtab PMS SDZ FRS TEV 0.5104 0.5104 0.5104 0.5104 02377616 Apo-Montelukast 5mg chewtab APX 0.5635 02379325 02380757 02354985 02330393 02238216 02355515 02374609 02391422 02379236 02379333 02368226 02373947 02328593 02238217 02355523 Montelukast 5mg chewtab MYLAN-Montelukast 5mg chewtab pms-Montelukast 5mg chewtab Sandoz Montelukast 5mg chewtab Singulair 5mg chewtab Teva-Montelukast 5mg chewtab Apo-Montelukast 10mg tab Jamp-Montelukast 10mg tab Montelukast 10mg tab (AHI) Montelukast 10mg tab (SAS) MYLAN-Montelukast 10mg tab pms-Montelukast FC 10mg tab Sandoz Montelukast 10mg tab Singulair 10mg tab Teva-Montelukast 10mg tab SAS MYL PMS SDZ FRS TEV APX JPC AHI SAS MYL PMS SDZ FRS TEV 0.5635 0.5635 0.5635 0.5635 0.5635 0.5635 0.8276 0.8276 0.8276 0.8276 0.8276 0.8276 0.8276 0.8276 0.8276 02350815 02015439 02302764 02244790 02350890 02014297 02302772 02244791 02350912 02014300 02302780 02245286 02244792 02350920 02014319 02302799 02350947 02014327 02302802 Morphine SR 15mg tab MS Contin 15mg SR tab Novo-Morphine 15mg SR tab Sandoz Morphine 15mg SR tab Morphine SR 30mg tab MS Contin 30mg SR tab Novo-Morphine 30mg SR tab Sandoz Morphine 30mg SR tab Morphine SR 60mg tab MS Contin 60mg SR tab Novo-Morphine 60mg SR tab pms-Morphine Sulfate 60mg SR tab (discontinued) Sandoz Morphine 60mg SR tab Morphine SR 100mg tab MS Contin 100mg SR tab Novo-Morphine 100mg SR tab Morphine SR 200mg tab MS Contin 200mg SR tab Novo-Morphine 200mg SR tab SAS PFR TEV SDZ SAS PFR TEV SDZ SAS PFR TEV PMS SDZ SAS PFR TEV SAS PFR TEV 0.2317 0.2317 0.2317 0.2317 0.3500 0.3500 0.3500 0.3500 0.6167 0.6167 0.6167 0.6167 0.6167 0.9401 0.9401 0.9401 1.7479 1.7479 1.7479 00392588 00392561 01916947 Morphine Sulfate 10mg/mL inj Morphine Sulfate 15mg/mL inj Bactroban 2% oint SDZ SDZ GSK 0.9900 1.0050 0.3453 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 53 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength mupirocin 2% oint DIN 02279983 Brand Taro-Mupirocin 2% oint MFR MRP TAR 0.3453 nabilone 0.5mg cap (exception status) 02256193 02393581 02380900 02358085 02384884 00548375 02393603 02380919 02358093 02384892 Cesamet 0.5mg cap CO Nabilone 0.5mg cap pms-Nabilone 0.5mg cap RAN-Nabilone 0.5mg cap Teva-Nabilone 0.5mg cap Cesamet 1mg cap CO Nabilone 1mg cap pms-Nabilone 1mg cap RAN-Nabilone 1mg cap Teva-Nabilone 1mg cap VLN COB PMS RAN TEV VLN COB PMS RAN TEV 02238639 02244563 02240867 02240868 Apo-Nabumetone 500mg tab MYLAN-Nabumetone 500mg tab Novo-Nabumetone 500mg tab Novo-Nabumetone 750mg tab APX MYL TEV TEV 00782505 02126753 00782467 02126761 00782475 00522678 00522651 02350750 00565350 Apo-Nadol 40mg tab Novo-Nadolol 40mg tab Apo-Nadol 80mg tab Novo-Nadolol 80mg tab Apo-Nadol 160mg tab Apo-Naproxen 125mg tab Apo-Naproxen 250mg tab Naproxen 250mg tab Teva-Naproxen 250mg tab APX TEV APX TEV APX APX APX SAS TEV 0.2465 0.2465 0.3515 0.3515 1.2046 0.0781 0.1068 0.1068 0.1068 naproxen 375mg tab 00600806 02350769 00627097 Apo-Naproxen 375mg tab Naproxen 375mg tab Teva-Naproxen 375mg tab APX SAS TEV 0.1458 0.1458 0.1458 naproxen 500mg tab 00592277 02350777 00589861 Apo-Naproxen 500mg tab Naproxen 500mg tab Teva-Naproxen 500mg tab APX SAS TEV 0.2110 0.2110 0.2110 naproxen 250mg EC tab 02246699 02162792 02350785 02243312 02246700 02243432 02162415 02350793 02294702 02243313 Apo-Naproxen 250mg EC tab Naprosyn-E 250mg EC tab Naproxen 250mg EC tab Novo-Naprox 250mg EC tab Apo-Naproxen 375mg EC tab MYLAN-Naproxen 375mg EC tab Naprosyn-E 375mg EC tab Naproxen 375mg EC tab pms-Naproxen 375mg EC tab Teva-Naproxen-EC 375mg tab APX HLR SAS TEV APX MYL HLR SAS PMS TEV 0.1434 0.1434 0.1434 0.1434 0.1880 0.1880 0.1880 0.1880 0.1880 0.1880 02246701 02241024 02162423 02350807 Apo-Naproxen 500mg EC tab MYLAN-Naproxen 500mg EC tab Naprosyn-E 500mg EC tab Naproxen 500mg EC tab APX MYL HLR SAS 0.3396 0.3396 0.3396 0.3396 nabilone 1mg cap (exception status) nabumetone 500mg tab nabumetone 750mg tab nadolol 40mg tab nadolol 80mg tab nadolol 160mg tab naproxen 125mg tab naproxen 250mg tab naproxen 375mg EC tab naproxen 500mg EC tab 1.0859 1.0859 1.0859 1.0859 1.0859 2.1718 2.1718 2.1718 2.1718 2.1718 0.1750 0.1750 0.1750 0.3500 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 54 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength naproxen 500mg EC tab DIN 02243314 02294710 Brand Novo-Naprox 500mg EC tab pms-Naproxen 500mg EC tab MFR MRP TEV 0.3396 PMS 0.3396 naproxen sodium 275mg tab 02162725 00784354 02351013 00778389 02162717 01940309 02351021 02026600 Anaprox 275mg tab Apo-Napro-Na 275mg tab Naproxen Sodium 275mg tab Teva-Naproxen Sodium 275mg tab Anaprox DS 550mg tab Apo-Napro-Na DS 550mg tab Naproxen Sodium DS 550mg tab Teva-Naproxen Sodium DS 550mg tab HLR APX SAS TEV HLR APX SAS TEV 02017237 02237820 02314290 pms-Naproxen 500mg supp Amerge 1mg tab Novo-Naratriptan 1mg tab PMS GSK TEV 0.9639 7.7950 7.7950 naratriptan 2.5mg tab (exception status) 02237821 02314304 02322323 Amerge 2.5mg tab Novo-Naratriptan 2.5mg tab Sandoz Naratriptan 2.5mg tab GSK TEV SDZ 6.1438 6.1438 6.1438 nifedipine 5mg cap 00725110 00755907 02155907 02349167 Nifedipine 5mg cap Nifedipine 10mg tab Adalat XL 30mg tab MYLAN-Nifedipine 30mg ER tab AAP AAP BAY MYL 0.3992 0.5292 0.6171 0.6171 nifedipine 60mg ER tab 02155990 02321149 Adalat XL 60mg tab MYLAN-Nifedipine 60mg ER tab BAY MYL 0.9374 0.9374 nilotinib 150mg cap (exception status) 02368250 02315874 02231015 02231441 02238998 02220156 00778338 02240457 02177714 02220164 00778346 02240458 02177722 Tasigna 150mg cap Tasigna 200mg cap Novo-Furantoin 50mg cap Nitrolingual 0.4mg/dose pumpspray Rho-Nitro 0.4mg/dose pumpspray Apo-Nizatidine 150mg cap Axid 150mg cap Novo-Nizatidine 150mg cap pms-Nizatidine 150mg cap Apo-Nizatidine 300mg cap Axid 300mg cap Novo-Nizatidine 300mg cap pms-Nizatidine 300mg cap NVR NVR TEV SAV SDZ APX MMT TEV PMS APX MMT TEV PMS 02229524 02269627 02237682 02246596 02223511 00015229 02231781 02177692 02223538 Apo-Norflox 400mg tab CO Norfloxacin 400mg tab Novo-Norfloxacin 400mg tab pms-Norfloxacin 400mg tab Apo-Nortriptyline 10mg cap Aventyl 10mg cap Novo-Nortriptyline 10mg cap pms-Nortriptyline 10mg cap Apo-Nortriptyline 25mg cap APX COB TEV PMS APX PHL TEV PMS APX naproxen sodium 550mg tab naproxen 500mg supp naratriptan 1mg tab (exception status) nifedipine 10mg tab nifedipine 30mg ER tab nilotinib 200mg cap (exception status) nitrofurantoin 50mg cap nitroglycerin 0.4mg/dose pumpspray nizatidine 150mg cap nizatidine 300mg cap norfloxacin 400mg tab (exception status) nortriptyline 10mg cap nortriptyline 25mg cap 0.1750 0.1750 0.1750 0.1750 0.3500 0.3500 0.3500 0.3500 29.5926 42.0054 0.3984 0.0423 0.0423 0.1800 0.1800 0.1800 0.1800 0.3600 0.3600 0.3600 0.3600 0.7934 0.7934 0.7934 0.7934 0.0787 0.0787 0.0787 0.0787 0.1583 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 55 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength nortriptyline 25mg cap DIN 00015237 02231782 02177706 Brand Aventyl 25mg cap Novo-Nortriptyline 25mg cap pms-Nortriptyline 25mg cap MFR MRP PHL 0.1583 TEV 0.1583 PMS 0.1583 nystatin 100,000iu o/l 02194201 ratio-Nystatin 100,000iu/mL oral drops TEV 0.0740 octreotide 50mcg/mL inj 02248639 00839191 Octreotide Acetate Omega 50mcg/mL inj Sandostatin 50mcg/mL inj HOS NVR 2.2140 2.2140 octreotide 100mcg/mL inj 02248640 00839205 Octreotide Acetate Omega 100mcg/mL inj Sandostatin 100mcg/mL inj HOS NVR 4.1680 4.1680 octreotide 200mcg/mL inj 02248642 02049392 02248641 00839213 Octreotide Acetate Omega 200mcg/mL inj Sandostatin 200mcg/mL inj Octreotide Acetate Omega 500mcg/mL inj Sandostatin 500mcg/mL inj HOS 8.0220 NVR 8.0220 HOS 19.5960 NVR 19.5960 02231531 02231532 02248398 02143291 02252570 02247189 Ofloxacin 300mg tab Ofloxacin 400mg tab Apo-Ofloxacin 0.3% oph sol Ocuflox 0.3% oph sol pms-Ofloxacin 0.3% oph sol (discontinued) Sandoz Ofloxacin 0.3% oph sol AAP AAP APX ALL PMS SDZ 1.6625 1.6625 0.8561 0.8561 0.8561 0.8561 olanzapine 2.5mg tab (exception status) 02281791 02325659 02337878 02372819 02303116 02310341 02276712 02229250 Apo-Olanzapine 2.5mg tab CO Olanzapine 2.5mg tab MYLAN-Olanzapine 2.5mg tab Olanzapine 2.5mg tab pms-Olanzapine 2.5mg tab Sandoz Olanzapine 2.5mg tab Teva-Olanzapine 2.5mg tab Zyprexa 2.5mg tab APX COB MYL SAS PMS SDZ TEV LIL 0.6290 0.6290 0.6290 0.6290 0.6290 0.6290 0.6290 0.6290 olanzapine 5mg tab (exception status) 02281805 02325667 02337886 02372827 02303159 02310368 02276720 02229269 02281813 02325675 02337894 02372835 02303167 02310376 02276739 02229277 02281821 02325683 Apo-Olanzapine 5mg tab CO Olanzapine 5mg tab MYLAN-Olanzapine 5mg tab Olanzapine 5mg tab pms-Olanzapine 5mg tab Sandoz Olanzapine 5mg tab Teva-Olanzapine 5mg tab Zyprexa 5mg tab Apo-Olanzapine 7.5mg tab CO Olanzapine 7.5mg tab MYLAN-Olanzapine 7.5mg tab Olanzapine 7.5mg tab pms-Olanzapine 7.5mg tab Sandoz Olanzapine 7.5mg tab Teva-Olanzapine 7.5mg tab Zyprexa 7.5mg tab Apo-Olanzapine 10mg tab CO Olanzapine 10mg tab APX COB MYL SAS PMS SDZ TEV LIL APX COB MYL SAS PMS SDZ TEV LIL APX COB 1.2580 1.2580 1.2580 1.2580 1.2580 1.2580 1.2580 1.2580 1.8871 1.8871 1.8871 1.8871 1.8871 1.8871 1.8871 1.8871 2.5161 2.5161 octreotide 500mcg/mL inj ofloxacin 300mg tab (exception status) ofloxacin 400mg tab (exception status) ofloxacin 0.3% oph sol (exception status) olanzapine 7.5mg tab (exception status) olanzapine 10mg tab (exception status) Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 56 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength olanzapine 10mg tab (exception status) DIN 02337908 02372843 02303175 02310384 02276747 02229285 Brand MYLAN-Olanzapine 10mg tab Olanzapine 10mg tab pms-Olanzapine 10mg tab Sandoz Olanzapine 10mg tab Teva-Olanzapine 10mg tab Zyprexa 10mg tab MFR MRP MYL 2.5161 SAS 2.5161 PMS 2.5161 SDZ 2.5161 TEV 2.5161 LIL 2.5161 olanzapine 15mg tab (exception status) 02281848 02325691 02337916 02372851 02303183 02310392 02276755 02238850 02360616 Apo-Olanzapine 15mg tab CO Olanzapine 15mg tab MYLAN-Olanzapine 15mg tab Olanzapine 15mg tab pms-Olanzapine 15mg tab Sandoz Olanzapine 15mg tab Teva-Olanzapine 15mg tab Zyprexa 15mg tab Apo-Olanzapine ODT 5mg tab APX COB MYL SAS PMS SDZ TEV LIL APX 3.7741 3.7741 3.7741 3.7741 3.7741 3.7741 3.7741 3.7741 1.2511 02327562 02382709 02352974 02303191 02327775 02321343 02243086 CO Olanzapine ODT 5mg tab MYLAN-Olanzapine ODT 5mg tab Olanzapine ODT 5mg tab pms-Olanzapine ODT 5mg tab Sandoz Olanzapine ODT 5mg tab Teva-Olanzapine OD 5mg tab Zyprexa Zydis 5mg tab COB MYL SAS PMS SDZ TEV LIL 1.2511 1.2511 1.2511 1.2511 1.2511 1.2511 1.2511 02360624 Apo-Olanzapine ODT 10mg tab APX 2.5000 02327570 02382717 02352982 02303205 02327783 02321351 02243087 CO Olanzapine ODT 10mg tab MYLAN-Olanzapine ODT 10mg tab Olanzapine ODT 10mg tab pms-Olanzapine ODT 10mg tab Sandoz Olanzapine ODT 10mg tab Teva-Olanzapine OD 10mg tab Zyprexa Zydis 10mg tab COB MYL SAS PMS SDZ TEV LIL 2.5000 2.5000 2.5000 2.5000 2.5000 2.5000 2.5000 02360632 Apo-Olanzapine ODT 15mg tab APX 3.7489 02327589 02382725 02352990 02303213 02327791 02321378 02243088 02360640 CO Olanzapine ODT 15mg tab MYLAN-Olanzapine ODT 15mg tab Olanzapine ODT 15mg tab pms-Olanzapine ODT 15mg tab Sandoz Olanzapine ODT 15mg tab Teva-Olanzapine OD 15mg tab Zyprexa Zydis 15mg tab Apo-Olanzapine ODT 20mg tab COB MYL SAS PMS SDZ TEV LIL APX 3.7489 3.7489 3.7489 3.7489 3.7489 3.7489 3.7489 5.9376 02327597 02382733 02327805 CO Olanzapine ODT 20mg tab MYLAN-Olanzapine ODT 20mg tab Sandoz Olanzapine ODT 20mg tab COB MYL SDZ 5.9376 5.9376 5.9376 olanzapine ODT 5mg tab (exception status) olanzapine ODT 10mg tab (exception status) olanzapine ODT 15mg tab (exception status) olanzapine ODT 20mg tab (exception status) Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 57 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength olanzapine ODT 20mg tab (exception status) DIN 02321386 Brand Teva-Olanzapine OD 20mg tab MFR MRP TEV 5.9376 02243089 Zyprexa Zydis 20mg tab LIL omeprazole 10mg cap 02119579 02329425 02296438 Losec 10mg cap MYLAN-Omeprazole 10mg cap Sandoz Omeprazole 10mg cap AZE MYL SDZ omeprazole 20mg cap 02245058 00846503 02329433 02348691 02320851 02296446 Apo-Omeprazole 20mg cap Losec 20mg cap MYLAN-Omeprazole 20mg cap Omeprazole 20mg cap pms-Omeprazole 20mg cap Sandoz Omeprazole 20mg cap APX AZE MYL SAS PMS SDZ omeprazole 10mg cap/tab 02230737 02329425 02245058 02190915 02329433 02295415 02348691 02310260 02374870 02260867 02288184 02296349 02313685 02371731 02305259 02297868 02264056 02306212 02278618 02258188 02312247 02278529 02274310 02376091 02213567 Losec 10mg tab MYLAN-Omeprazole 10mg cap Apo-Omeprazole 20mg cap Losec 20mg tab MYLAN-Omeprazole 20mg cap Novo-Omeprazole Delayed-Release 20mg tab Omeprazole 20mg cap pms-Omeprazole DR 20mg tab RAN-Omeprazole 20mg tab ratio-Omeprazole 20mg tab Apo-Ondansetron 4mg tab CO Ondansetron 4mg tab Jamp-Ondansetron 4mg tab Mar-Ondansetron 4mg tab MINT- Ondansetron 4mg tab MYLAN-Ondansetron 4mg tab Novo-Ondansetron 4mg tab Ondansetron-Odan 4mg tab phl-Ondansetron 4mg tab pms-Ondansetron 4mg tab RAN-Ondansetron 4mg tab ratio-Ondansetron 4mg tab Sandoz Ondansetron 4mg tab Septa-Ondansetron 4mg tab Zofran 4mg tab AZE MYL APX AZE MYL TEV SAS PMS RAN TEV APX COB JPC MAR MNT MYL TEV ODN PHL PMS RAN TEV SDZ SPT GSK 02288192 02296357 02313693 02371758 02305267 02297876 02306220 02278626 Apo-Ondansetron 8mg tab CO Ondansetron 8mg tab Jamp-Ondansetron 8mg tab Mar-Ondansetron 8mg tab MINT-Ondansetron 8mg tab MYLAN-Ondansetron 8mg tab Ondansetron-Odan 8mg tab phl-Ondansetron 8mg tab APX COB JPC MAR MNT MYL ODN PHL omeprazole 20mg cap/tab ondansetron 4mg tab (exception status) ondansetron 8mg tab (exception status) 5.9376 0.2059 0.2059 0.2059 0.4117 0.4117 0.4117 0.4117 0.4117 0.4117 0.2059 0.2059 0.4117 0.4117 0.4117 0.4117 0.4117 0.4117 0.4117 0.4117 3.5778 3.5778 3.5778 3.5778 3.5778 3.5778 3.5778 3.5778 3.5778 3.5778 3.5778 3.5778 3.5778 3.5778 3.5778 7.1555 7.1555 7.1555 7.1555 7.1555 7.1555 7.1555 7.1555 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 58 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength ondansetron 8mg tab (exception status) DIN 02258196 02312255 02278537 02274329 02376105 02264064 02213575 Brand pms-Ondansetron 8mg tab RAN-Ondansetron 8mg tab ratio-Ondansetron 8mg tab Sandoz Ondansetron 8mg tab Septa-Ondansetron 8mg tab Teva-Ondansetron 8mg tab Zofran 8mg tab MFR MRP PMS 7.1555 RAN 7.1555 TEV 7.1555 SDZ 7.1555 SPT 7.1555 TEV 7.1555 GSK 7.1555 ondansetron 4mg ODT tab (exception status) ondansetron 8mg ODT tab (exception status) ondansetron 4mg/5mL o/l (exception status) 02239372 Zofran 4mg ODT tab GSK 3.5778 02239373 Zofran 8mg ODT tab GSK 7.1555 02291967 Ondansetron 4mg/5mL o/l AAP 1.5856 02229639 Zofran 4mg/5mL o/l GSK 1.5856 02236783 00402745 00402737 Apo-Orciprenaline 2mg/mL syr Apo-Oxazepam 15mg tab Apo-Oxazepam 30mg tab APX APX APX 0.0574 0.0560 0.0764 02284294 Apo-Oxcarbazepine 150mg tab APX 0.6209 02242067 Trileptal 150mg tab NVR 0.6209 02284308 Apo-Oxcarbazepine 300mg tab APX 0.9102 02242068 Trileptal 300mg tab NVR 0.9102 02284316 Apo-Oxcarbazepine 600mg tab APX 1.8204 02242069 02163543 02230800 02230394 02350238 02240550 02223376 Trileptal 600mg tab Apo-Oxybutynin 5mg tab MYLAN-Oxybutynin 5mg tab Novo-Oxybutynin 5mg tab Oxybutynin 5mg tab pms-Oxbytynin 5mg tab pms-Oxybutynin 1mg/mL o/l NVR APX MYL TEV SAS PMS PMS 1.8204 0.1508 0.1508 0.1508 0.1508 0.1508 0.1183 02319977 00789739 02319985 00443948 02231934 02319977 02240131 02319985 02240132 02319993 02292912 pms-Oxycodone 5mg tab Supeudol 5mg tab pms-Oxycodone 10mg tab Supeudol 10mg tab Oxy-IR 5mg tab pms-Oxycodone 5mg tab Oxy-IR 10mg tab pms-Oxycodone 10mg tab Oxy-IR 20mg tab pms-Oxycodone 20mg tab Apo-Pantoprazole 20mg DR tab PMS SDZ PMS SDZ PFR PMS PFR PMS PFR PMS APX 0.1776 0.1776 0.2760 0.2760 0.1776 0.1776 0.2760 0.2760 0.4538 0.4538 02241804 02305038 Pantoloc 20mg DR tab RAN-Pantoprazole 20mg DR tab NYC RAN orciprenaline 2mg/mL syr oxazepam 15mg tab oxazepam 30mg tab oxcarbazepine 150mg tab (exception status) oxcarbazepine 300mg tab (exception status) oxcarbazepine 600mg tab (exception status) oxybutynin 5mg tab oxybutynin 1mg/mL o/l oxycodone 5mg tab (Supeudol) oxycodone 10mg tab (Supeudol) oxycodone 5mg tab (Oxy-IR) oxycodone 10mg tab (Oxy-IR) oxycodone 20mg tab pantoprazole 20mg EC tab (exception status) Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 59 of 87 PRP 0.3538 0.3538 0.3538 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength pantoprazole 20mg EC tab (exception status) DIN 02301075 Brand Sandoz Pantoprazole 20mg DR tab MFR MRP SDZ 02285479 Teva-Pantoprazole 20mg DR tab TEV 02292920 Apo-Pantoprazole 40mg DR tab APX 0.7076 02300486 02299585 02229453 02370808 02307871 02305046 02301083 02285487 CO Pantoprazole 40mg DR tab MYLAN-Pantoprazole 40mg DR tab Pantoloc 40mg DR tab Pantoprazole 40mg tab pms-Pantoprazole 40mg DR tab RAN-Pantoprazole 40mg DR tab Sandoz Pantoprazole 40mg DR tab Teva-Pantoprazole 40mg DR tab COB MYL NYC SAS PMS RAN SDZ TEV 0.7076 0.7076 0.7076 0.7076 0.7076 0.7076 0.7076 0.7076 paroxetine 20mg tab 02240908 02383284 02262754 02368870 02248013 02282852 01940481 02248451 02247751 02247811 02269430 02248557 Apo-Paroxetine 20mg tab Auro-Paroxetine 20mg tab CO Paroxetine 20mg tab Jamp-Paroxetine 20mg tab MYLAN-Paroxetine 20mg tab Paroxetine 20mg tab Paxil 20mg tab phl-Paroxetine 20mg tab pms-Paroxetine 20mg tab ratio-Paroxetine 20mg tab Sandoz Paroxetine 20mg tab Teva-Paroxetine 20mg tab APX ARO COB JPC MYL SAS GSK PHL PMS TEV SDZ TEV 0.6320 0.6320 0.6320 0.6320 0.6320 0.6320 0.6320 0.6320 0.6320 0.6320 0.6320 0.6320 paroxetine 30mg tab 02240909 02383292 02262762 02368889 02248014 02282860 01940473 02248452 02247752 02247812 02269449 02248558 02352303 02371448 Apo-Paroxetine 30mg tab Auro-Paroxetine 30mg tab CO Paroxetine 30mg tab Jamp-Paroxetine 30mg tab MYLAN-Paroxetine 30mg tab Paroxetine 30mg tab Paxil 30mg tab phl-Paroxetine 30mg tab pms-Paroxetine 30mg tab ratio-Paroxetine 30mg tab Sandoz Paroxetine 30mg tab Teva-Paroxetine 30mg tab Votrient 200mg tab Victrelis Triple 80mcg Inj/200mg/200mg cap APX ARO COB JPC MYL SAS GSK PHL PMS TEV SDZ TEV GSK FRS 0.6714 0.6714 0.6714 0.6714 0.6714 0.6714 0.6714 0.6714 0.6714 0.6714 0.6714 0.6714 peginterferon alfa-2B 100mcg, boceprevir 200mg & ribavirin 200mg kit (exception status) 02371456 Victrelis Triple 100mcg Inj/200mg/200mg cap FRS 2878.0168 peginterferon alfa-2B 120mcg, boceprevir 200mg & ribavirin 200mg kit (exception status) 02371464 Victrelis Triple 120mcg Inj/200mg/200mg cap FRS 2957.7100 pantoprazole 40mg EC tab (exception status) pazopanib 200mg tab (exception status) peginterferon alfa-2B 80mcg, boceprevir 200mg & ribavirin 200mg kit (exception status) PRP 0.3538 0.3538 37.0000 2878.0168 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 60 of 87 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength peginterferon alfa-2B 150mcg, boceprevir 200mg & ribavirin 200mg kit (exception status) DIN 02371472 Brand Victrelis Triple 1500mcg Inj/200mg/200mg cap MFR MRP FRS penicillin V potassium 300mg tab 00642215 00021202 Apo-Pen VK 300mg tab Novo-Pen-VK 300mg tab APX TEV 0.0710 0.0710 penicillin V potassium 60mg/mL o/l 00642231 00391603 Apo-Pen VK 60mg/mL o/l Novo-Pen-VK 60mg/mL o/l APX TEV 0.0472 0.0472 pentoxifylline 400mg tab (exception status) 02230090 Apo-Pentoxifylline 400mg SR tab APX 0.5846 02221977 Trental 400mg tab SAV 0.5846 pethidine 75mg/mL inj 00335126 00725765 00725757 Perphenazine 4mg tab Meperidine 50mg/mL inj Meperidine 75mg/mL inj AAP SDZ SDZ 0.0823 0.9600 1.0100 pethidine 100mg/mL inj 00725749 Meperidine 100mg/mL inj SDZ 1.0700 phenylephrine 10mg/mL inj 02241980 01953583 00023450 02250896 02245432 00313815 02245433 00313823 Neo-Synephrine 10mg/mL inj Phenylephrine 10mg/mL inj Dilantin-125 25mg/mL susp Taro-Phenytoin 25mg/mL susp Apo-Pimozide 2mg tab Orap 2mg tab Apo-Pimozide 4mg tab Orap 4mg tab HOS SDZ PFI TAR APX PHL APX PHL 4.4300 4.4300 0.0311 0.0311 0.3093 0.3093 0.4136 0.4136 00755877 00869007 02231536 02261782 00417270 00755885 00869015 02231537 02261790 00443174 Apo-Pindol 5mg tab Novo-Pindol 5mg tab pms-Pindolol 5mg tab Sandoz Pindolol 5mg tab Visken 5mg tab Apo-Pindol 10mg tab Novo-Pindol 10mg tab pms-Pindolol 10mg tab Sandoz Pindolol 10mg tab Visken 10mg tab APX TEV PMS SDZ NVR APX TEV PMS SDZ NVR 0.2050 0.2050 0.2050 0.2050 0.2050 0.3500 0.3500 0.3500 0.3500 0.3500 00755893 00869023 02231539 02261804 00417289 02242572 02302942 02384906 02302861 02326477 02298279 02274914 02307669 Apo-Pindol 15mg tab Novo-Pindol 15mg tab pms-Pindolol 15mg tab Sandoz Pindolol 15mg tab Visken 15mg tab Actos 15mg tab Apo-Pioglitazone 15mg tab Auro-Pioglitazone 15mg tab CO Pioglitazone 15mg tab MINT-Pioglitazone 15mg tab MYLAN-Pioglitazone 15mg tab Novo-Pioglitazone 15mg tab phl-Pioglitazone 15mg tab APX TEV PMS SDZ NVR LIL APX ARO COB MNT MYL TEV PHL 0.5078 0.5078 0.5078 0.5078 0.5078 0.8324 0.8324 0.8324 0.8324 0.8324 0.8324 0.8324 0.8324 perphenazine 4mg tab pethidine 50mg/mL inj phenytoin 25mg/mL susp pimozide 2mg tab pimozide 4mg tab pindolol 5mg tab pindolol 10mg tab pindolol 15mg tab pioglitazone 15mg tab (exception status) PRP 2957.7100 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 61 of 87 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength pioglitazone 15mg tab (exception status) pioglitazone 30mg tab (exception status) pioglitazone 45mg tab (exception status) piroxicam 10mg cap piroxicam 20mg cap piroxicam 20mg supp polymixin b sulfate, neomycin sulfate & gramicidin oph/otic sol polymyxin b sulfate, neomycin sulfate & hydrocortisone otic sol potassium chloride 1.33mEq/mL o/l DIN 02303124 Brand pms-Pioglitazone 15mg tab MFR MRP PMS 0.8324 02375850 02301423 02297906 02320754 RAN-Pioglitazone 15mg tab ratio-Pioglitazone 15mg tab Sandoz Pioglitazone 15mg tab Zym-Pioglitazone 15mg tab RAN TEV SDZ ZYM 0.8324 0.8324 0.8324 0.8324 02242573 02302950 02384914 02302888 02326485 02298287 02274922 02307677 02339587 02303132 02375869 02301431 02297914 02320762 02242574 02302977 02384922 02302896 02326493 02298295 02274930 02307723 02339595 02303140 02375877 02301458 02297922 02320770 00642886 00695718 Actos 30mg tab Apo-Pioglitazone 30mg tab Auro-Pioglitazone 30mg tab CO Pioglitazone 30mg tab MINT-Pioglitazone 30mg tab MYLAN-Pioglitazone 30mg tab Novo-Pioglitazone 30mg tab phl-Pioglitazone 30mg tab Pioglitazone 30mg tab pms-Pioglitazone 30mg tab RAN-Pioglitazone 30mg tab ratio-Pioglitazone 30mg tab (discontinued) Sandoz Pioglitazone 30mg tab Zym-Pioglitazone 30mg tab Actos 45mg tab Apo-Pioglitazone 45mg tab Auro-Pioglitazone 45mg tab CO Pioglitazone 45mg tab MINT-Pioglitazone 45mg tab MYLAN-Pioglitazone 45mg tab Novo-Pioglitazone 45mg tab phl-Pioglitazone 45mg tab Pioglitazone 45mg tab pms-Pioglitazone 45mg tab RAN-Pioglitazone 45mg tab ratio-Pioglitazone 45mg tab Sandoz Pioglitazone 45mg tab Zym-Pioglitazone 45mg tab Apo-Piroxicam 10mg cap Novo-Pirocam 10mg cap LIL APX ARO COB MNT MYL TEV PHL AHI PMS RAN TEV SDZ ZYM LIL APX ARO COB MNT MYL TEV PHL AHI PMS RAN TEV SDZ ZYM APX TEV 1.1662 1.1662 1.1662 1.1662 1.1662 1.1662 1.1662 1.1662 1.1662 1.1662 1.1662 1.1662 1.1662 1.1662 1.7535 1.7535 1.7535 1.7535 1.7535 1.7535 1.7535 1.7535 1.7535 1.7535 1.7535 1.7535 1.7535 1.7535 0.3211 0.3211 00642894 00695696 02154463 Apo-Piroxicam 20mg cap Novo-Pirocam 20mg cap pms-Piroxicam 20mg supp APX TEV PMS 0.5196 0.5196 2.2329 00807435 Optimyxin Plus oph/otic sol (discontinued) SDZ 0.8230 01912828 Cortisporin otic sol GSK 1.1400 02230386 Sandoz Cortimyxin otic sol SDZ 1.1400 80024360 K-10 1.33mEq/mL o/l GSK 0.0158 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 62 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength potassium chloride 1.33mEq/mL o/l DIN 02238604 Brand pms-Potassium Chloride 1.33mEq/mL o/l MFR MRP PMS 0.0158 pramipexole 0.25mg tab 02292378 02297302 02237145 02376350 02269309 02290111 02315262 Apo-Pramipexole 0.25mg tab CO Pramipexole 0.25mg tab Mirapex 0.25mg tab MYLAN-Pramipexole 0.25mg tab Novo-Pramipexole 0.25mg tab pms-Pramipexole 0.25mg tab Sandoz Pramipexole 0.25mg tab APX COB BOE MYL TEV PMS SDZ 0.3680 0.3680 0.3680 0.3680 0.3680 0.3680 0.3680 pramipexole 1mg tab 02292394 02297329 02237146 02376377 02269325 02290146 02315289 02292408 02297337 02237147 02376385 02269333 02290154 02315297 Apo-Pramipexole 1mg tab CO Pramipexole 1mg tab Mirapex 1mg tab MYLAN-Pramipexole 1mg tab Novo-Pramipexole 1mg tab pms-Pramipexole 1mg tab Sandoz Pramipexole 1mg tab Apo-Pramipexole 1.5mg tab CO Pramipexole 1.5mg tab Mirapex 1.5mg tab MYLAN-Pramipexole 1.5mg tab Novo-Pramipexole 1.5mg tab pms-Pramipexole 1.5mg tab Sandoz Pramipexole 1.5mg tab APX COB BOE MYL TEV PMS SDZ APX COB BOE MYL TEV PMS SDZ 0.7360 0.7360 0.7360 0.7360 0.7360 0.7360 0.7360 0.7360 0.7360 0.7360 0.7360 0.7360 0.7360 0.7360 02243506 02248182 02330954 02317451 02257092 02247008 02249766 02247655 00893749 02356546 02284421 02247856 02243507 02248183 02330962 02317478 02257106 02247009 02249774 02247656 00893757 02356554 02284448 Apo-Pravastatin 10mg tab CO Pravastatin 10mg tab Jamp-Pravastatin 10mg tab MINT-Pravastatin 10mg tab MYLAN-Pravastatin 10mg tab Novo-Pravastatin 10mg tab phl-Pravastatin 10mg tab pms-Pravastatin 10mg tab Pravachol 10mg tab Pravastatin 10mg tab RAN-Pravastatin 10mg tab Sandoz Pravastatin 10mg tab Apo-Pravastatin 20mg tab CO Pravastatin 20mg tab Jamp-Pravastatin 20mg tab MINT-Pravastatin 20mg tab MYLAN-Pravastatin 20mg tab Novo-Pravastatin 20mg tab phl-Pravastatin 20mg tab pms-Pravastatin 20mg tab Pravachol 20mg tab Pravastatin 20mg tab RAN-Pravastatin 20mg tab APX COB JPC MNT MYL TEV PHL PMS BRI SAS RAN SDZ APX COB JPC MNT MYL TEV PHL PMS BRI SAS RAN 0.5670 0.5670 0.5670 0.5670 0.5670 0.5670 0.5670 0.5670 0.5670 0.5670 0.5670 0.5670 0.6689 0.6689 0.6689 0.6689 0.6689 0.6689 0.6689 0.6689 0.6689 0.6689 0.6689 pramipexole 1.5mg tab pravastatin 10mg tab pravastatin 20mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 63 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength pravastatin 20mg tab DIN 02247857 Brand Sandoz Pravastatin 20mg tab MFR MRP SDZ 0.6689 pravastatin 40mg tab 02243508 02248184 02330970 02317486 02257114 02247010 02249782 02247657 02222051 02356562 02284456 02247858 00882801 01934198 00882828 01934201 Apo-Pravastatin 40mg tab CO Pravastatin 40mg tab Jamp-Pravastatin 40mg tab MINT-Pravastatin 40mg tab MYLAN-Pravastatin 40mg tab Novo-Pravastatin 40mg tab phl-Pravastatin 40mg tab pms-Pravastatin 40mg tab Pravachol 40mg tab Pravastatin 40mg tab RAN-Pravastatin 40mg tab Sandoz Pravastatin 40mg tab Apo-Prazo 1mg tab Novo-Prazin 1mg tab Apo-Prazo 2mg tab Novo-Prazin 2mg tab APX COB JPC MNT MYL TEV PHL PMS BRI SAS RAN SDZ APX TEV APX TEV 0.8057 0.8057 0.8057 0.8057 0.8057 0.8057 0.8057 0.8057 0.8057 0.8057 0.8057 0.8057 0.1371 0.1371 0.1862 0.1862 prazosin HCl 5mg tab 00882836 01934228 Apo-Prazo 5mg tab Novo-Prazin 5mg tab APX TEV 0.2560 0.2560 prednisolone acetate 1% oph susp 00301175 00700401 01916203 Pred Forte 1% oph susp ratio-Prednisolone 1% oph susp Sandoz Prednisolone 1% oph susp ALL TEV SDZ 1.9400 1.9400 1.9400 prednisolone sodium phosphate 1mg/mL o/l 02230619 Pediapred oral sol SAV 0.0936 prazosin HCl 1mg tab prazosin HCl 2mg tab 02245532 pms-Prednisolone oral sol PMS 0.0936 prednisone 1mg tab 00598194 00271373 Apo-Prednisone 1mg tab Winpred 1mg tab APX VLN 0.1072 0.1072 prednisone 5mg tab 00312770 00021695 Apo-Prednisone 5mg tab Novo-Prednisone 5mg tab APX TEV 0.0401 0.0401 prednisone 50mg tab 00550957 00232378 Apo-Prednisone 50mg tab Novo-Prednisone 50mg tab APX TEV 0.1735 0.1735 primidone 125mg tab primidone 250mg tab prochlorperazine 5mg tab 00399310 Primidone 125mg tab AAP 0.0600 00396761 00886440 00886432 00789747 00587354 Primidone 250mg tab Apo-Prochlorazine 5mg tab Apo-Prochlorazine 10mg tab Prochlorperazine 5mg/mL inj pms-Procyclidine 5mg tab AAP APX APX SDZ PMS 0.0944 0.1659 0.2025 1.0450 0.1396 00587362 02243324 02245372 02294559 02343053 00603708 02243325 pms-Procyclidine 0.5mg/mL elx Apo-Propafenone 150mg tab MYLAN-Propafenone 150mg tab pms-Propafenone 150mg tab Propafenone 150mg tab Rythmol 150mg tab Apo-Propafenone 300mg tab PMS APX MYL PMS SAS ABB APX 0.2730 0.4227 0.4227 0.4227 0.4227 0.4227 0.7450 prochlorperazine 10mg tab prochlorperazine 5mg/mL inj procyclidine HCl 5mg tab procyclidine HCl 0.5mg/mL o/l propafenone 150mg tab propafenone 300mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 64 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength propafenone 300mg tab DIN 02245373 02294575 02343061 00603716 Brand MYLAN-Propafenone 300mg tab pms-Propafenone 300mg tab Propafenone 300mg tab Rythmol 300mg tab MFR MRP MYL 0.7450 PMS 0.7450 SAS 0.7450 ABB 0.7450 propranolol 10mg tab 00402788 00496480 00663719 00740675 Apo-Propranolol 10mg tab Novo-Pranol 10mg tab Apo-Propranolol 20mg tab Novo-Pranol 20mg tab APX TEV APX TEV 0.0192 0.0192 0.0346 0.0346 00402753 00496499 00402761 00496502 00504335 Apo-Propranolol 40mg tab Novo-Pranol 40mg tab Apo-Propranolol 80mg tab Novo-Pranol 80mg tab Apo-Propranolol 120mg tab APX TEV APX TEV APX 0.0348 0.0348 0.0585 0.0585 0.3091 02313901 02316080 02330415 02307804 02284235 02299054 02296551 02353164 02313995 02236951 02313928 02316099 02330423 02307812 02284243 02299062 02296578 02353172 02314002 02236952 02313936 02316110 02330458 02307839 02284278 02299089 02296594 02353199 02314010 02236953 Apo-Quetiapine 25mg tab CO Quetiapine 25mg tab Jamp-Quetiapine 25mg tab MYLAN-Quetiapine 25mg tab Novo-Quetiapine 25mg tab phl-Quetiapine 25mg tab pms-Quetiapine 25mg tab Quetiapine 25mg tab Sandoz Quetiapine 25mg tab Seroquel 25mg tab Apo-Quetiapine 100mg tab CO Quetiapine 100mg tab Jamp-Quetiapine 100mg tab MYLAN-Quetiapine 100mg tab Novo-Quetiapine 100mg tab phl-Quetiapine 100mg tab pms-Quetiapine 100mg tab Quetiapine 100mg tab Sandoz Quetiapine 100mg tab Seroquel 100mg tab Apo-Quetiapine 200mg tab CO Quetiapine 200mg tab Jamp-Quetiapine 200mg tab MYLAN-Quetiapine 200mg tab Novo-Quetiapine 200mg tab phl-Quetiapine 200mg tab pms-Quetiapine 200mg tab Quetiapine 200mg tab Sandoz Quetiapine 200mg tab Seroquel 200mg tab APX COB JPC MYL TEV PHL PMS SAS SDZ AZE APX COB JPC MYL TEV PHL PMS SAS SDZ AZE APX COB JPC MYL TEV PHL PMS SAS SDZ AZE 0.1779 0.1779 0.1779 0.1779 0.1779 0.1779 0.1779 0.1779 0.1779 0.1779 0.4746 0.4746 0.4746 0.4746 0.4746 0.4746 0.4746 0.4746 0.4746 0.4746 0.9530 0.9530 0.9530 0.9530 0.9530 0.9530 0.9530 0.9530 0.9530 0.9530 02313944 Apo-Quetiapine 300mg tab APX 1.3906 propranolol 20mg tab propranolol 40mg tab propranolol 80mg tab propranolol 120mg tab quetiapine 25mg tab quetiapine 100mg tab quetiapine 200mg tab quetiapine 300mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 65 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength quetiapine 300mg tab DIN 02316129 02330466 02307847 02284286 02299097 02296608 02353202 02314029 02244107 Brand CO Quetiapine 300mg tab Jamp-Quetiapine 300mg tab MYLAN-Quetiapine 300mg tab Novo-Quetiapine 300mg tab phl-Quetiapine 300mg tab pms-Quetiapine 300mg tab Quetiapine 300mg tab Sandoz Quetiapine 300mg tab Seroquel 300mg tab MFR MRP COB 1.3906 JPC 1.3906 MYL 1.3906 TEV 1.3906 PHL 1.3906 PMS 1.3906 SAS 1.3906 SDZ 1.3906 AZE 1.3906 quinine sulfate 200mg cap 00021008 00021016 Novo-Quinine 200mg cap Novo-Quinine 300mg cap TEV TEV 0.2481 0.3893 rabeprazole 10mg EC tab 02345579 02243796 02381737 02310805 02356511 02298074 02314177 02296632 Apo-Rabeprazole 10mg EC tab Pariet 10mg EC tab PAT-Rabeprazole 10mg tab pms-Rabeprazole 10mg EC tab Rabeprazole EC 10mg tab RAN-Rabeprazole 10mg EC tab Sandoz Rabeprazole 10mg EC tab Teva-Rabeprazole-EC 10mg tab APX JAN PPH PMS SAS RAN SDZ TEV 0.1204 0.1204 0.1204 0.1204 0.1204 0.1204 0.1204 0.1204 rabeprazole 20mg EC tab 02345587 02243797 02381745 02310813 02356538 02298082 02314185 02296640 Apo-Rabeprazole 20mg EC tab Pariet 20mg EC tab PAT-Rabeprazole 20mg tab pms-Rabeprazole 20mg EC tab Rabeprazole EC 20mg tab RAN-Rabeprazole 20mg EC tab Sandoz Rabeprazole 20mg EC tab Teva-Rabeprazole-EC 20mg tab APX JAN PPH PMS SAS RAN SDZ TEV 0.2408 0.2408 0.2408 0.2408 0.2408 0.2408 0.2408 0.2408 raloxifene 60mg tab (exception status) 02279215 02358840 02239028 02312298 02358921 02221829 02251515 02295482 02331101 02301148 02295369 02310503 02291398 02221837 02251531 02295490 02331128 02301156 Apo-Raloxifene 60mg tab CO Raloxifene 60mg tab Evista 60mg tab Novo-Raloxifene 60mg tab pms-Raloxifene 60mg tab Altace 1.25mg cap Apo-Ramipril 1.25mg cap CO Ramipril 1.25mg cap Jamp-Ramipril 1.25mg cap MYLAN-Ramipril 1.25mg cap pms-Ramipril 1.25mg cap RAN-Ramipril 1.25mg cap Sandoz Ramipril 1.25mg tab Altace 2.5mg cap Apo-Ramipril 2.5mg cap CO Ramipril 2.5mg cap Jamp-Ramipril 2.5mg cap MYLAN-Ramipril 2.5mg cap APX COB LIL TEV PMS SAV APX COB JPC MYL PMS RAN SDZ SAV APX COB JPC MYL 0.8457 0.8457 0.8457 0.8457 0.8457 0.1274 0.1274 0.1274 0.1274 0.1274 0.1274 0.1274 0.1274 0.1470 0.1470 0.1470 0.1470 0.1470 quinine sulfate 300mg cap ramipril 1.25mg cap/tab ramipril 2.5mg cap/tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 66 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength ramipril 2.5mg cap/tab DIN 02247917 02374846 02310511 02291401 02247945 Brand pms-Ramipril 2.5mg cap Ramipril 2.5mg cap RAN-Ramipril 2.5mg cap Sandoz Ramipril 2.5mg tab Teva-Ramipril 2.5mg cap MFR MRP PMS 0.1470 SAS 0.1470 RAN 0.1470 SDZ 0.1470 TEV 0.1470 ramipril 5mg cap/tab 02221845 02251574 02295504 02331136 02301164 02247918 02374854 02310538 02291428 02247946 Altace 5mg cap Apo-Ramipril 5mg cap CO Ramipril 5mg cap Jamp-Ramipril 5mg cap MYLAN-Ramipril 5mg cap pms-Ramipril 5mg cap Ramipril 5mg cap RAN-Ramipril 5mg cap Sandoz Ramipril 5mg tab Teva-Ramipril 5mg cap SAV APX COB JPC MYL PMS SAS RAN SDZ TEV 0.1470 0.1470 0.1470 0.1470 0.1470 0.1470 0.1470 0.1470 0.1470 0.1470 ramipril 10mg cap/tab 02221853 02251582 02295512 02331144 02301172 02247919 02374862 02310546 02291436 02247947 Altace 10mg cap Apo-Ramipril 10mg cap CO Ramipril 10mg cap Jamp-Ramipril 10mg cap MYLAN-Ramipril 10mg cap pms-Ramipril 10mg cap Ramipril 10mg cap RAN-Ramipril 10mg cap Sandoz Ramipril 10mg tab Teva-Ramipril 10mg cap SAV APX COB JPC MYL PMS SAS RAN SDZ TEV 0.1862 0.1862 0.1862 0.1862 0.1862 0.1862 0.1862 0.1862 0.1862 0.1862 ramipril 15mg cap 02281112 02325381 02283131 Altace 15mg cap Apo-Ramipril 15mg cap Altace HCT 2.5/12.5mg tab SAV APX SAV 0.8132 0.8132 0.2250 02342138 02283158 pms-Ramipril-HCTZ 2.5/12.5mg tab Altace HCT 5/12.5mg tab PMS SAV 0.2250 0.2263 02342146 pms-Ramipril-HCTZ 5/12.5mg tab PMS 0.2263 02283174 Altace HCT 5/25mg tab SAV 0.2263 02342162 02283166 pms-Ramipril-HCTZ 5/25mg tab Altace HCT 10/12.5mg tab PMS SAV 0.2263 0.2865 02342154 02283182 pms-Ramipril-HCTZ 10/12.5mg tab Altace HCT 10/25mg tab PMS SAV 0.2865 0.2865 02342170 00733059 02248570 02367378 02207761 pms-Ramipril-HCTZ 10/25mg tab Apo-Ranitidine 150mg tab CO Ranitidine 150mg tab Myl-Ranitidine 150mg tab MYLAN-Ranitidine 150mg tab PMS APX COB MYL MYL 0.2865 0.1800 0.1800 0.1800 0.1800 ramipril 2.5mg & hydrochlorothiazide 12.5mg tab ramipril 5mg & hydrochlorothiazide 12.5mg tab ramipril 5mg & hydrochlorothiazide 25mg tab ramipril 10mg & hydrochlorothiazide 12.5mg tab ramipril 10mg & hydrochlorothiazide 25mg tab ranitidine 150mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 67 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength ranitidine 150mg tab DIN 00828564 02242453 02336480 02353016 00828823 02243229 02212331 Brand Novo-Ranidine 150mg tab pms-Ranitidine 150mg tab RAN-Ranitidine 150mg tab Ranitidine 150mg tab ratio-Ranitidine 150mg tab Sandoz Ranitidine 150mg tab Zantac 150mg tab MFR MRP TEV 0.1800 PMS 0.1800 RAN 0.1800 SAS 0.1800 TEV 0.1800 SDZ 0.1800 GSK 0.1800 ranitidine 300mg tab 00733067 02248571 02367386 02207788 00828556 02242454 02336502 02353024 02243230 02212358 02256711 02212366 02280833 02242940 02091887 00393444 Apo-Ranitidine 300mg tab CO Ranitidine 300mg tab Myl-Ranitidine 300mg tab MYLAN-Ranitidine 300mg tab Novo-Ranidine 300mg tab pms-Ranitidine 300mg tab RAN-Ranitidine 300mg tab Ranitidine 300mg tab Sandoz Ranitidine 300mg tab Zantac 300mg tab Ranitidine 25mg/mL inj Zantac 25mg/mL inj Apo-Ranitidine 15mg/mL o/l Novo-Ranidine 15mg/mL o/l Rifadin 150mg cap Rofact 150mg cap APX COB MYL MYL TEV PMS RAN SAS SDZ GSK SDZ GSK APX TEV SAV VLN 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 1.3310 1.3310 0.0932 0.0932 0.6552 0.6552 rifampin 300mg cap 02092808 00343617 Rifadin 300mg cap Rofact 300mg cap SAV VLN 1.0311 1.0311 riluzole 50mg tab (exception status) 02352583 02390299 02242763 Apo-Riluzole 50mg tab MYLAN-Riluzole 50mg tab Rilutek 50mg tab APX MYL SAV 3.4361 3.4361 3.4361 risedronate 5mg tab (exception status) 02242518 02298376 Actonel 5mg tab Teva-Risedronate 5mg tab WNC TEV 1.3897 1.3897 risedronate 30mg tab (exception status) 02239146 02298384 02246896 02353687 02368552 02357984 02302209 02319861 02370255 02327295 02298392 02282119 02282585 02359529 Actonel 30mg tab Teva-Risedronate 30mg tab Actonel 35mg tab Apo-Risedronate 35mg tab Jamp-Risedronate 35mg tab MYLAN-Risedronate 35mg tab pms-Risedronate 35mg tab ratio-Risedronate 35mg tab Risedronate 35mg tab Sandoz-Risedronate 35mg tab Teva-Risedronate 35mg tab Apo-Risperidone 0.25mg tab CO Risperidone 0.25mg tab Jamp-Risperidone 0.25mg tab WNC TEV WNC APX JPC MYL PMS TEV SAS SDZ TEV APX COB JPC 9.0033 9.0033 4.1300 4.1300 4.1300 4.1300 4.1300 4.1300 4.1300 4.1300 4.1300 0.1840 0.1840 0.1840 ranitidine 25mg/mL inj ranitidine 15mg/mL o/l rifampin 150mg cap risedronate 35mg tab (exception status) risperidone 0.25mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 68 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength risperidone 0.25mg tab risperidone 0.5mg tab risperidone 1mg tab risperidone 2mg tab DIN 02371766 02359790 02282240 02282690 02258439 02252007 02328305 02240551 02356880 02303655 02282127 02282593 02359537 02371774 02359804 02282259 02264188 02258447 02252015 02328313 02240552 02356899 02303663 Brand Mar-Risperidone 0.25mg tab MINT Risperidone 0.25mg tab MYLAN-Risperidone 0.25mg tab Novo-Risperidone 0.25mg tab phl-Risperidone 0.25mg tab pms-Risperidone 0.25mg tab RAN-Risperidone 0.25mg tab Risperdal 0.25mg tab Risperidone 0.25mg tab Sandoz Risperidone 0.25mg tab Apo-Risperidone 0.5mg tab CO Risperidone 0.5mg tab Jamp-Risperidone 0.5mg tab Mar-Risperidone 0.5mg tab MINT Risperidone 0.5mg tab MYLAN-Risperidone 0.5mg tab Novo-Risperidone 0.5mg tab phl-Risperidone 0.5mg tab pms-Risperidone 0.5mg tab RAN-Risperidone 0.5mg tab Risperdal 0.5mg tab Risperidone 0.5mg tab Sandoz Risperidone 0.5mg tab MFR MRP MAR 0.1840 MNT 0.1840 MYL 0.1840 TEV 0.1840 PHL 0.1840 PMS 0.1840 RAN 0.1840 JAN 0.1840 SAS 0.1840 SDZ 0.1840 APX 0.3082 COB 0.3082 JPC 0.3082 MAR 0.3082 MNT 0.3082 MYL 0.3082 TEV 0.3082 PHL 0.3082 PMS 0.3082 RAN 0.3082 JAN 0.3082 SAS 0.3082 SDZ 0.3082 02282135 02282607 02359545 02371782 02359812 02282267 02264196 02258455 02252023 02328321 02025280 02356902 02279800 02282143 02282615 02359553 02371790 02359820 02282275 02264218 02258463 02252031 Apo-Risperidone 1mg tab CO Risperidone 1mg tab Jamp-Risperidone 1mg tab Mar-Risperidone 1mg tab MINT Risperidone 1mg tab MYLAN-Risperidone 1mg tab Novo-Risperidone 1mg tab phl-Risperidone 1mg tab pms-Risperidone 1mg tab RAN-Risperidone 1mg tab Risperdal 1mg tab Risperidone 1mg tab Sandoz Risperidone 1mg tab Apo-Risperidone 2mg tab CO Risperidone 2mg tab Jamp-Risperidone 2mg tab Mar-Risperidone 2mg tab MINT-Risperidone 2mg tab MYLAN-Risperidone 2mg tab Novo-Risperidone 2mg tab phl-Risperidone 2mg tab pms-Risperidone 2mg tab APX COB JPC MAR MNT MYL TEV PHL PMS RAN JAN SAS SDZ APX COB JPC MAR MNT MYL TEV PHL PMS 0.4258 0.4258 0.4258 0.4258 0.4258 0.4258 0.4258 0.4258 0.4258 0.4258 0.4258 0.4258 0.4258 0.8500 0.8500 0.8500 0.8500 0.8500 0.8500 0.8500 0.8500 0.8500 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 69 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength risperidone 2mg tab DIN 02328348 02025299 02356910 02279819 Brand RAN-Risperidone 2mg tab Risperdal 2mg tab Risperidone 2mg tab Sandoz Risperidone 2mg tab MFR MRP RAN 0.8500 JAN 0.8500 SAS 0.8500 SDZ 0.8500 risperidone 3mg tab 02282151 02282623 02359561 02371804 02359839 02282283 02264226 02258471 02252058 02328364 02025302 02356929 02279827 02282178 02282631 02359588 02371812 02359847 02282291 02264234 02258498 02252066 02328372 02025310 02356937 02279835 02291789 02247705 Apo-Risperidone 3mg tab CO Risperidone 3mg tab Jamp-Risperidone 3mg tab Mar-Risperidone 3mg tab MINT Risperidone 3mg tab MYLAN-Risperidone 3mg tab Novo-Risperidone 3mg tab phl-Risperidone 3mg tab pms-Risperidone 3mg tab RAN-Risperidone 3mg tab Risperdal 3mg tab Risperidone 3mg tab Sandoz Risperidone 3mg tab Apo-Risperidone 4mg tab CO Risperidone 4mg tab Jamp-Risperidone 4mg tab Mar-Risperidone 4mg tab MINT Risperidone 4mg tab MYLAN-Risperidone 4mg tab Novo-Risperidone 4mg tab phl-Risperidone 4mg tab pms-Risperidone 4mg tab RAN-Risperidone 4mg tab Risperdal 4mg tab Risperidone 4mg tab Sandoz Risperidone 4mg tab pms-Risperidone ODT 1mg tab Risperdal M-tab (1mg) APX COB JPC MAR MNT MYL TEV PHL PMS RAN JAN SAS SDZ APX COB JPC MAR MNT MYL TEV PHL PMS RAN JAN SAS SDZ PMS JAN 1.2751 1.2751 1.2751 1.2751 1.2751 1.2751 1.2751 1.2751 1.2751 1.2751 1.2751 1.2751 1.2751 1.7001 1.7001 1.7001 1.7001 1.7001 1.7001 1.7001 1.7001 1.7001 1.7001 1.7001 1.7001 1.7001 0.7727 0.7727 02291797 02247706 02370697 02268086 02370700 02268094 02280396 02279266 02236950 02241927 02336715 02242115 pms-Risperidone ODT 2mg tab Risperdal M-tab (2mg) pms-Risperidone ODT 3mg tab Risperdal M-tab (3mg) pms-Risperidone ODT 4mg tab Risperdal M-tab (4mg) Apo-Risperidone 1mg/mL o/l pms-Risperidone 1mg/mL o/l Risperdal 1mg/mL o/l Rituxan 10mg/mL inj Apo-Rivastigmine 1.5mg cap Exelon 1.5mg cap PMS JAN PMS JAN PMS JAN APX PMS JAN HLR APX NVR 1.5280 1.5280 2.2913 2.2913 3.0638 3.0638 0.4802 0.4802 0.4802 risperidone 4mg tab risperidone ODT 1mg tab risperidone ODT 2mg tab risperidone ODT 3mg tab risperidone ODT 4mg tab risperidone 1mg/mL o/l rituximab 10mg/mL inj (exception status) rivastigmine 1.5mg cap (exception status) 55.6063 0.9121 0.9121 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 70 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength rivastigmine 1.5mg cap (exception status) rivastigmine 3mg cap (exception status) rivastigmine 4.5mg cap (exception status) rivastigmine 6mg cap (exception status) rizatriptan 5mg tab (exception status) rizatriptan 10mg tab (exception status) rizatriptan ODT 5mg tab (exception status) rizatriptan ODT 10mg tab (exception status) DIN 02332809 Brand MYLAN-Rivastigmine 1.5mg cap MFR MRP MYL 0.9121 02305984 02306034 02311283 02324563 Novo-Rivastigmine 1.5mg cap pms-Rivastigmine 1.5mg cap ratio-Rivastigmine 1.5mg cap Sandoz Rivastigmine 1.5mg cap TEV PMS TEV SDZ 0.9121 0.9121 0.9121 0.9121 02336723 02242116 02332817 02305992 02306042 02311291 02324571 02336731 02242117 02332825 02306018 02306050 02311305 02324598 02336758 02242118 02332833 02306026 02306069 02311313 02324601 02380455 02379651 02381702 02380463 02379678 02240521 Apo-Rivastigmine 3mg cap Exelon 3mg cap MYLAN-Rivastigmine 3mg cap Novo-Rivastigmine 3mg cap pms-Rivastigmine 3mg cap ratio-Rivastigmine 3mg cap Sandoz Rivastigmine 3mg cap Apo-Rivastigmine 4.5mg cap Exelon 4.5mg cap MYLAN-Rivastigmine 4.5mg cap Novo-Rivastigmine 4.5mg cap pms-Rivastigmine 4.5mg cap ratio-Rivastigmine 4.5mg cap Sandoz Rivastigmine 4.5mg cap Apo-Rivastigmine 6mg cap Exelon 6mg cap MYLAN-Rivastigmine 6mg cap Novo-Rivastigmine 6mg cap pms-Rivastigmine 6mg cap (discontinued) ratio-Rivastigmine 6mg cap Sandoz Rivastigmine 6mg cap Jamp-Rizatriptan 5mg tab Mar-Rizatriptan 5mg tab CO Rizatriptan 10mg tab Jamp-Rizatriptan 10mg tab Mar-Rizatriptan 10mg tab Maxalt 10mg tab APX NVR MYL TEV PMS TEV SDZ APX NVR MYL TEV PMS TEV SDZ APX NVR MYL TEV PMS TEV SDZ JPC MAR COB JPC MAR FRS 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 0.9121 5.1870 5.1870 5.1870 5.1870 5.1870 5.1870 02374730 CO Rizatriptan ODT 5mg tab COB 5.1870 02240518 02379198 02393360 02351870 02374749 Maxalt RPD 5mg wafers MYLAN-Rizatriptan ODT 5mg tab pms-Rizatriptan RDT 5mg tab Sandoz Rizatriptan ODT 5mg tab CO Rizatriptan ODT 10mg tab FRS MYL PMS SDZ COB 5.1870 5.1870 5.1870 5.1870 5.1870 02240519 02379201 02393379 02351889 Maxalt RPD 10mg wafers MYLAN-Rizatriptan ODT 10mg tab pms-Rizatriptan RDT 10mg tab Sandoz Rizatriptan ODT 10mg tab FRS MYL PMS SDZ 5.1870 5.1870 5.1870 5.1870 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 71 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength ropinirole 0.25mg tab DIN 02316846 02352338 02326590 02314037 02232565 02353040 Brand CO Ropinirole 0.25mg tab Jamp-Ropinirole 0.25mg tab pms-Ropinirole 0.25mg tab RAN-Ropinirole 0.25mg tab ReQuip 0.25mg tab Ropinirole 0.25mg tab MFR MRP COB 0.0993 JPC 0.0993 PMS 0.0993 RAN 0.0993 GSK 0.0993 SAS 0.0993 ropinirole 1mg tab 02316854 02352346 02326612 02314053 02232567 02353059 02316862 02352354 02326620 02314061 02232568 02353067 CO Ropinirole 1mg tab Jamp-Ropinirole 1mg tab pms-Ropinirole 1mg tab RAN-Ropinirole 1mg tab ReQuip 1mg tab Ropinirole 1mg tab CO Ropinirole 2mg tab Jamp-Ropinirole 2mg tab pms-Ropinirole 2mg tab RAN-Ropinirole 2mg tab ReQuip 2mg tab Ropinirole 2mg tab COB JPC PMS RAN GSK SAS COB JPC PMS RAN GSK SAS 0.3974 0.3974 0.3974 0.3974 0.3974 0.3974 0.4371 0.4371 0.4371 0.4371 0.4371 0.4371 ropinirole 5mg tab 02316870 02352362 02326639 02314088 02232569 02353075 CO Ropinirole 5mg tab Jamp-Ropinirole 5mg tab pms-Ropinirole 5mg tab RAN-Ropinirole 5mg tab ReQuip 5mg tab Ropinirole 5mg tab COB JPC PMS RAN NVR SAS 1.2034 1.2034 1.2034 1.2034 1.2034 1.2034 rosuvastatin 10mg tab 02337983 02339773 02247162 02391260 02381273 02378531 02382652 02338734 02354616 Apo-Rosuvastatin 10mg tab CO Rosuvastatin 10mg tab Crestor 10mg tab Jamp-Rosuvastatin 10mg tab MYLAN-Rosuvastatin 10mg tab pms-Rosuvastatin 10mg tab RAN-Rosuvastatin 10mg tab Sandoz Rosuvastatin 10mg tab Teva-Rosuvastatin 10mg tab APX COB AZE JPC MYL PMS RAN SDZ TEV 0.4760 0.4760 0.4760 0.4760 0.4760 0.4760 0.4760 0.4760 0.4760 rosuvastatin 20mg tab 02337991 02339781 02247163 02391279 02381281 02378558 02382660 02338742 02354624 02338009 02339803 02247164 Apo-Rosuvastatin 20mg tab CO Rosuvastatin 20mg tab Crestor 20mg tab Jamp-Rosuvastatin 20mg tab MYLAN-Rosuvastatin 20mg tab pms-Rosuvastatin 20mg tab RAN-Rosuvastatin 20mg tab Sandoz Rosuvastatin 20mg tab Teva-Rosuvastatin 20mg tab Apo-Rosuvastatin 40mg tab CO Rosuvastatin 40mg tab Crestor 40mg tab APX COB AZE JPC MYL PMS RAN SDZ TEV APX COB AZE 0.5950 0.5950 0.5950 0.5950 0.5950 0.5950 0.5950 0.5950 0.5950 0.6965 0.6965 0.6965 ropinirole 2mg tab rosuvastatin 40mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 72 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength rosuvastatin 40mg tab DIN 02391287 02381303 02378566 02382679 02338750 02354632 Brand Jamp-Rosuvastatin 40mg tab MYLAN-Rosuvastatin 40mg tab pms-Rosuvastatin 40mg tab RAN-Rosuvastatin 40mg tab Sandoz Rosuvastatin 40mg tab Teva-Rosuvastatin 40mg tab MFR MRP JPC 0.6965 MYL 0.6965 PMS 0.6965 RAN 0.6965 SDZ 0.6965 TEV 0.6965 salbutamol 100mcg/dose oral inh 02232570 02245669 02326450 02241497 02069571 Airomir 100mcg/dose oral inh Apo-Salvent CFC Free 100mcg/dose oral inh Novo-Salbutamol HFA 100mcg/dose oral inh Ventolin HFA 100mcg/dose oral inh pms-Salbutamol 5mg/mL inh sol 10mL MDS APX TEV GSK PMS 0.0325 0.0325 0.0325 0.0325 0.3511 00860808 02154412 02213486 ratio-Salbutamol 5mg/mL inh sol 10mL Sandoz Salbutamol 5mg/mL inh sol 10mL Ventolin 5mg/mL inh sol 10mL TEV SDZ GSK 0.3511 0.3511 0.3511 02146843 02146851 Apo-Salvent 2mg tab Apo-Salvent 4mg tab APX APX 0.1274 0.2134 02208245 pms-Salbutamol 0.5mg/mL UD inh sol PMS 0.0293 02239365 ratio-Salbutamol 0.5mg/mL UD inh sol TEV 0.0293 01926934 MYLAN-Salbutamol 1mg/mL UD inh sol MYL 0.0585 02208229 01986864 02213419 pms-Salbutamol 1mg/mL UD inh sol ratio-Salbutamol 1mg/mL UD inh sol Ventolin 1mg/mL UD inh sol PMS TEV GSK 0.0585 0.0585 0.0585 02173360 MYLAN-Salbutamol 2mg/mL UD inh sol MYL 0.1170 02208237 02239366 02213427 pms-Salbutamol 2mg/mL UD inh sol ratio-Salbutamol 2mg/mL UD inh sol Ventolin 2mg/mL UD inh sol PMS TEV GSK 0.1170 0.1170 0.1170 02229868 02230641 02231036 02068087 02238280 02287390 02273683 02357143 02242519 02240485 02245824 02244838 02374552 02245159 02353520 02132702 Hyoscine Butylbromide 20mg/mL inj Apo-Selegiline 5mg tab MYLAN-Selegiline 5mg tab Novo-Selegiline 5mg tab Apo-Sertraline 25mg cap CO Sertraline 25mg cap GD-Sertraline 25mg cap Jamp-Sertraline 25mg cap MYLAN-Sertraline 25mg cap Novo-Sertraline 25mg cap phl-Sertraline 25mg cap pms-Sertraline 25mg cap RAN-Sertraline 25mg cap Sandoz Sertraline 25mg cap Sertraline 25mg cap Zoloft 25mg cap SDZ APX MYL TEV APX COB GMD JPC MYL TEV PHL PMS RAN SDZ SAS PFI salbutamol 5mg/mL inh sol (exception status) salbutamol 2mg tab salbutamol 4mg tab salbutamol 0.5mg/mL unit dose inh sol (exception status) salbutamol 1mg/mL unit dose inh sol (exception status) salbutamol 2mg/mL unit dose inh sol (exception status) scopolamine 20mg/mL inj selegiline 5mg tab sertraline 25mg cap 4.5150 0.7030 0.7030 0.7030 0.2814 0.2814 0.2814 0.2814 0.2814 0.2814 0.2814 0.2814 0.2814 0.2814 0.2814 0.2814 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 73 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength sertraline 50mg cap DIN 02238281 02287404 02273691 02357151 02242520 02240484 02245825 02244839 02374560 02245160 02353539 01962817 Brand Apo-Sertraline 50mg cap CO Sertraline 50mg cap GD-Sertraline 50mg cap Jamp-Sertraline 50mg cap MYLAN-Sertraline 50mg cap Novo-Sertraline 50mg cap phl-Sertraline 50mg cap pms-Sertraline 50mg cap RAN-Sertraline 50mg cap Sandoz Sertraline 50mg cap Sertraline 50mg cap Zoloft 50mg cap MFR MRP APX 0.5628 COB 0.5628 GMD 0.5628 JPC 0.5628 MYL 0.5628 TEV 0.5628 PHL 0.5628 PMS 0.5628 RAN 0.5628 SDZ 0.5628 SAS 0.5628 PFI 0.5628 sertraline 100mg cap 02238282 02287412 02273705 02357178 02242521 02245826 02244840 02374579 02245161 02353547 02240481 01962779 02319500 02279401 02247011 02248103 02375591 02331020 02375036 02372932 02246582 02281546 02269252 02329131 02284723 02250144 00884324 02247012 02248104 02375605 02331039 02375044 02372940 Apo-Sertraline 100mg cap CO Sertraline 100mg cap GD-Sertraline 100mg cap Jamp-Sertraline 100mg cap MYLAN-Sertraline 100mg cap phl-Sertraline 100mg cap pms-Sertraline 100mg cap RAN-Sertraline 100mg cap Sandoz Sertraline 100mg cap Sertraline 100mg cap Teva-Sertraline 100mg cap Zoloft 100mg cap ratio-Sildenafil-R 20mg tab Revatio 20mg tab Apo-Simvastatin 5mg tab CO Simvastatin 5mg tab Jamp-Simvastatin 5mg tab Jamp-Simvastatin 5mg tab (discontinued) Mar-Simvastatin 5mg tab MINT-Simvastatin 5mg tab MYLAN-Simvastatin 5mg tab phl-Simvastatin 5mg tab pms-Simvastatin 5mg tab RAN-Simvastatin 5mg tab Simvastatin 5mg tab Teva-Simvastatin 5mg tab Zocor 5mg tab Apo-Simvastatin 10mg tab CO Simvastatin 10mg tab Jamp-Simvastatin 10mg tab Jamp-Simvastatin 10mg tab (discontinued) Mar-Simvastatin 10mg tab MINT-Simvastatin 10mg tab APX COB GMD JPC MYL PHL PMS RAN SDZ SAS TEV PFI TEV PFI APX COB JPC JPC MAR MNT MYL PHL PMS RAN SAS TEV FRS APX COB JPC JPC MAR MNT sildenafil 20mg tab simvastatin 5mg tab simvastatin 10mg tab 0.5898 0.5898 0.5898 0.5898 0.5898 0.5898 0.5898 0.5898 0.5898 0.5898 0.5898 0.5898 7.4399 7.4399 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.3600 0.7081 0.7081 0.7081 0.7081 0.7081 0.7081 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 74 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength simvastatin 10mg tab DIN 02246583 02250152 02281554 02269260 02329158 02247828 02284731 00884332 Brand MYLAN-Simvastatin 10mg tab Novo-Simvastatin 10mg tab phl-Simvastatin 10mg tab pms-Simvastatin 10mg tab RAN-Simvastatin 10mg tab Sandoz Simvastatin 10mg tab Simvastatin 10mg tab Zocor 10mg tab MFR MRP MYL 0.7081 TEV 0.7081 PHL 0.7081 PMS 0.7081 RAN 0.7081 SDZ 0.7081 SAS 0.7081 FRS 0.7081 simvastatin 20mg tab 02247013 02248105 02375613 02331047 02375052 02372959 02246737 02250160 02281562 02269279 02329166 02247830 02284758 00884340 02247014 02248106 02375621 02331055 02375060 02372967 02246584 02281570 02269287 02329174 02247831 02284766 02250179 00884359 02247015 02248107 02375648 02331063 02375079 02246585 02281589 02269295 02329182 Apo-Simvastatin 20mg tab CO Simvastatin 20mg tab Jamp-Simvastatin 20mg tab Jamp-Simvastatin 20mg tab (discontinued) Mar-Simvastatin 20mg tab MINT-Simvastatin 20mg tab MYLAN-Simvastatin 20mg tab Novo-Simvastatin 20mg tab phl-Simvastatin 20mg tab pms-Simvastatin 20mg tab RAN-Simvastatin 20mg tab Sandoz Simvastatin 20mg tab Simvastatin 20mg tab Zocor 20mg tab Apo-Simvastatin 40mg tab CO Simvastatin 40mg tab Jamp-Simvastatin 40mg tab Jamp-Simvastatin 40mg tab (discontinued) Mar-Simvastatin 40mg tab MINT-Simvastatin 40mg tab MYLAN-Simvastatin 40mg tab phl-Simvastatin 40mg tab pms-Simvastatin 40mg tab RAN-Simvastatin 40mg tab Sandoz Simvastatin 40mg tab Simvastatin 40mg tab Teva-Simvastatin 40mg tab Zocor 40mg tab Apo-Simvastatin 80mg tab CO Simvastatin 80mg tab Jamp-Simvastatin 80mg tab Jamp-Simvastatin 80mg tab (discontinued) Mar-Simvastatin 80mg tab MYLAN-Simvastatin 80mg tab phl-Simvastatin 80mg tab pms-Simvastatin 80mg tab RAN-Simvastatin 80mg tab APX COB JPC JPC MAR MNT MYL TEV PHL PMS RAN SDZ SAS FRS APX COB JPC JPC MAR MNT MYL PHL PMS RAN SDZ SAS TEV FRS APX COB JPC JPC MAR MYL PHL PMS RAN simvastatin 40mg tab simvastatin 80mg tab 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 0.8751 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 75 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength simvastatin 80mg tab DIN 02247833 02284774 02250187 02240332 Brand Sandoz Simvastatin 80mg tab Simvastatin 80mg tab Teva-Simvastatin 80mg tab Zocor 80mg tab MFR MRP SDZ 0.8751 SAS 0.8751 TEV 0.8751 FRS 0.8751 sodium aurothiomalate 10mg/mL inj 01927620 02245456 02245457 Myochrysine 10mg/mL inj Sodium Aurothiomalate 10mg/mL inj Sodium Aurothiomalate 25mg/mL inj SAV SDZ SDZ 01927604 02245458 02284227 02210428 02270625 02368617 02229778 02231181 02238326 02084228 02257831 02167794 02270633 02368625 02229779 02231182 02238327 02084236 00028606 00613215 00285455 00613223 Myochrysine 50mg/mL inj Sodium Aurothiomalate 50mg/mL inj Nexavar 200mg tab Apo-Sotalol 80mg tab CO Sotalol 80mg tab (discontinued) Jamp-Sotalol 80mg tab MYLAN-Sotalol 80mg tab Novo-Sotalol 80mg tab pms-Sotalol 80mg tab ratio-Sotalol 80mg tab Sandoz Sotalol 80mg tab Apo-Sotalol 160mg tab CO Sotalol 160mg tab (discontinued) Jamp-Sotalol 160mg tab MYLAN-Sotalol 160mg tab Novo-Sotalol 160mg tab pms-Sotalol 160mg tab ratio-Sotalol 160mg tab Aldactone 25mg tab Novo-Spiroton 25mg tab Aldactone 100mg tab Novo-Spiroton 100mg tab SAV 18.2100 SDZ 18.2100 BAY APX 0.2966 COB 0.2966 JPC 0.2966 MYL 0.2966 TEV 0.2966 PMS 0.2966 TEV 0.2966 SDZ 0.2966 APX 0.2273 COB 0.2273 JPC 0.2273 MYL 0.2273 TEV 0.2273 PMS 0.2273 TEV 0.2273 PFI 0.1057 TEV 0.1057 PFI 0.2461 TEV 0.2461 02125250 02045702 02100622 02244147 00445274 Apo-Sucralfate 1g tab Novo-Sucralate 1g tab Sulcrate 1g tab Sufentanil Citrate 50mcg/mL inj Apo-Sulfatrim 400/80mg tab APX TEV AXC SDZ APX 0.1924 0.1924 0.1924 6.8300 0.0482 00510637 00445282 Novo-Trimel 400/80mg tab Apo-Sulfatrim 800/160mg DS tab TEV APX 0.0482 0.1221 00510645 Novo-Trimel 800/160mg DS tab TEV 0.1221 00726540 Novo-Trimel 40/8mg susp TEV 0.0929 00441767 Sulfinpyrazone 200mg tab AAP 0.3252 00778354 00745588 00778362 Apo-Sulin 150mg tab Novo-Sundac 150mg tab Apo-Sulin 200mg tab APX TEV APX sodium aurothiomalate 25mg/mL inj sodium aurothiomalate 50mg/mL inj sorafenib 200mg tab (exception status) sotalol 80mg tab sotalol 160mg tab spironolactone 25mg tab spironolactone 100mg tab sucralfate 1g tab sufentanil citrate 50mcg/mL inj sulfamethoxazole 400mg & trimethoprim 80mg tab sulfamethoxazole 800mg & trimethoprim 160mg tab sulfamethoxazole 40mg & trimethoprim 8mg/mL o/l sulfinpyrazone 200mg tab sulindac 150mg tab sulindac 200mg tab 9.6600 9.6600 11.7100 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 76 of 87 PRP 48.8928 0.3500 0.3500 0.3500 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 1 Generic Name and Strength sulindac 200mg tab DIN 00745596 Brand Novo-Sundac 200mg tab MFR MRP TEV sumatriptan 50mg tab (exception status) 02268388 02257890 02212153 02268914 02286823 02256436 02263025 02286521 Apo-Sumatriptan 50mg tab CO Sumatriptan 50mg tab Imitrex DF 50mg tab MYLAN-Sumatriptan 50mg tab Novo-Sumatriptan DF 50mg tab pms-Sumatriptan 50mg tab Sandoz Sumatriptan 50mg tab Sumatriptan 50mg tab APX COB GSK MYL TEV PMS SDZ SAS 7.1350 7.1350 7.1350 7.1350 7.1350 7.1350 7.1350 7.1350 sumatriptan 100mg tab (exception status) 02268396 02257904 02212161 02268922 02239367 02286831 02256444 02263033 02286548 Apo-Sumatriptan 100mg tab CO Sumatriptan 100mg tab Imitrex DF 100mg tab MYLAN-Sumatriptan 100mg tab Novo-Sumatriptan 100mg tab Novo-Sumatriptan DF 100mg tab pms-Sumatriptan 100mg tab Sandoz Sumatriptan 100mg tab Sumatriptan 100mg tab APX COB GSK MYL TEV TEV PMS SDZ SAS 7.8600 7.8600 7.8600 7.8600 7.8600 7.8600 7.8600 7.8600 7.8600 sumatriptan 12mg/mL inj (exception status) 02212188 Imitrex 6mg/0.5mL inj refill cartridge GSK 61.7200 00999446 00901886 02361698 Imitrex 6mg/0.5mL inj stat dose kit Imitrex 6mg/0.5mL inj unit dose Sumatriptan SUN 6mg/0.5mL inj GSK 61.7200 GSK 61.7200 TAR 61.7200 sunitinib 12.5mg cap (exception status) sunitinib 25mg cap (exception status) 02280795 02280809 Sutent 12.5mg cap Sutent 25mg cap PFI PFI sunitinib 50mg cap (exception status) 02280817 Sutent 50mg cap PFI tamoxifen citrate 10mg tab 00812404 02088428 00851965 Apo-Tamox 10mg tab MYLAN-Tamoxifen 10mg tab Novo-Tamoxifen 10mg tab APX MYL TEV 0.1750 0.1750 0.1750 tamoxifen citrate 20mg tab 00812390 02089858 02048485 00851973 Apo-Tamox 20mg tab MYLAN-Tamoxifen 20mg tab Nolvadex-D 20mg tab Novo-Tamoxifen 20mg tab APX MYL AZE TEV 0.3500 0.3500 0.3500 0.3500 telaprevir 375mg tab (exception status) 02371553 00999627 02393247 02240769 02376717 02391236 02375958 02388944 02320177 Incivek 375mg tab Incivek 375mg tab CO Telmisartan 40mg tab Micardis 40mg tab MYLAN-Telmisartan 40mg tab pms-Telmisartan 40mg tab Sandoz Telmisartan 40mg tab Telmisartan 40mg tab Teva-Telmisartan 40mg tab VTX VTX COB BOE MYL PMS SDZ SAS TEV 0.3954 0.3954 0.3954 0.3954 0.3954 0.3954 0.3954 02393255 02240770 02376725 CO Telmisartan 80mg tab Micardis 80mg tab MYLAN-Telmisartan 80mg tab COB BOE MYL 0.3954 0.3954 0.3954 telmisartan 40mg tab telmisartan 80mg tab 274.0807 75.2783 75.2783 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Page 77 of 87 PRP 0.3500 68.5207 137.0402 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. Version: NS Pharmacare Reimbursement List Effective April 2013 2 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength telmisartan 80mg tab DIN 02391244 02375966 02388952 02320185 Brand pms-Telmisartan 80mg tab Sandoz Telmisartan 80mg tab Telmisartan 80mg tab Teva-Telmisartan 80mg tab MFR MRP PMS 0.3954 SDZ 0.3954 SAS 0.3954 TEV 0.3954 telmisartan 80mg & hydrochlorothiazide 12.5mg tab 02393263 CO Telmisartan/HCT 80/12.5mg tab COB 0.3954 02244344 02373564 02393557 02395355 02330288 Micardis Plus 80/12.5mg tab MYLAN-Telmisartan HCTZ 80/12.5mg tab Sandoz Telmisartan HCTZ 80/12.5mg tab Telmisartan/HCTZ 80/12.5mg tab Teva-Telmisartan HCTZ 80/12.5mg tab BOE MYL SDZ SAS TEV 0.3954 0.3954 0.3954 0.3954 0.3954 02393271 CO Telisartan/HCT 80/25mg tab COB 0.3954 02318709 02373572 02393565 02395363 02379252 02225964 02244814 02230095 00604453 Micardis Plus 80/25mg tab MYLAN-Telmisartan HCTZ 80/25mg tab Sandoz Telmisartan HCTZ 80/25mg tab Telmisartan/HCTZ 80/25mg tab Teva-Telmisartan HCTZ 80/25mg tab Apo-Temazepam 15mg cap CO Temazepam 15mg cap Novo-Temazepam 15mg cap Restoril 15mg cap BOE MYL SDZ SAS TEV APX COB TEV ORX 0.3954 0.3954 0.3954 0.3954 0.3954 0.0699 0.0699 0.0699 0.0699 temazepam 30mg cap 02225972 02244815 02230102 00604461 Apo-Temazepam 30mg cap CO Temazepam 30mg cap Novo-Temazepam 30mg cap Restoril 30mg cap APX COB TEV ORX 0.0847 0.0847 0.0847 0.0847 temozolomide 20mg cap (exception status) temozolomide 100mg cap (exception status) temozolomide 140mg cap (exception status) temozolomide 250mg cap (exception status) tenoxicam 20mg tab terazosin 1mg tab 02241094 Temodal 20mg cap SCH 32.8680 02241095 Temodal 100mg cap SCH 160.9560 02312794 Temodal 140mg cap SCH 225.3400 02241096 Temodal 250mg cap SCH 402.3800 02230661 Tenoxicam 20mg tab AAP 0.7000 02234502 00818658 02243518 02218941 02350475 02230805 02234503 00818682 02243519 02218968 02350483 Apo-Terazosin 1mg tab Hytrin 1mg tab pms-Terazosin 1mg tab ratio-Terazosin 1mg tab Terazosin 1mg tab Teva-Terazosin 1mg tab Apo-Terazosin 2mg tab Hytrin 2mg tab pms-Terazosin 2mg tab ratio-Terazosin 2mg tab Terazosin 2mg tab APX ABB PMS TEV SAS TEV APX ABB PMS TEV SAS telmisartan 80mg & hydrochlorothiazide 25mg tab temazepam 15mg cap terazosin 2mg tab 0.2616 0.2616 0.2616 0.2616 0.2616 0.2616 0.3325 0.3325 0.3325 0.3325 0.3325 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 78 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength terazosin 2mg tab DIN 02230806 Brand Teva-Terazosin 2mg tab MFR MRP TEV 0.3325 terazosin 5mg tab 02234504 00818666 02243520 02218976 02350491 02230807 02234505 00818674 02243521 02218984 02350505 02230808 Apo-Terazosin 5mg tab Hytrin 5mg tab pms-Terazosin 5mg tab ratio-Terazosin 5mg tab Terazosin 5mg tab Teva-Terazosin 5mg tab Apo-Terazosin 10mg tab Hytrin 10mg tab pms-Terazosin 10mg tab ratio-Terazosin 10mg tab Terazosin 10mg tab Teva-Terazosin 10mg tab APX ABB PMS TEV SAS TEV APX ABB PMS TEV SAS TEV 0.4515 0.4515 0.4515 0.4515 0.4515 0.4515 0.6609 0.6609 0.6609 0.6609 0.6609 0.6609 terbinafine 250mg tab (exception status) 02239893 02320134 02254727 02352818 02357070 02031116 02242503 02240346 02294273 02262177 02353121 Apo-Terbinafine 250mg tab Auro-Terbinafine 250mg tab CO Terbinafine 250mg tab GD-Terbinafine 250mg tab Jamp-Terbinafine 250mg tab Lamisil 250mg tab MYLAN-Terbinafine 250mg tab Novo-Terbinafine 250mg tab pms-Terbinafine 250mg tab Sandoz Terbinafine 250mg tab Terbinafine 250mg tab APX ARO COB GMD JPC NVR MYL TEV PMS SDZ SAS 1.8526 1.8526 1.8526 1.8526 1.8526 1.8526 1.8526 1.8526 1.8526 1.8526 1.8526 testosterone cypionate 100mg/mL inj 00030783 02246063 00782327 02322498 Depo-Testosterone 100mg/mL inj Testosterone Cypionate 100mg/mL inj Andriol 40mg cap pms-Testosterone 40mg cap PFI SDZ SCH PMS 2.3580 2.3580 0.7650 0.7650 tetrabenazine 25mg tab 02199270 02402424 Nitoman 25mg tab pms-Tetrabenazine 25mg tab BVL PMS 4.8551 4.8551 tetracycline 250mg cap 00580929 02230086 02230087 02193221 02243525 02136112 02179679 02136120 02179687 02237701 02239744 02236848 02243587 02343045 Tetracycline 250mg cap Novo-Theophyl SR 200mg tab Novo-Theophyl SR 300mg tab Thiamiject 100mg/mL inj (OMG) Thiamine 100mg/mL inj Apo-Tiaprofenic 200mg tab Novo-Tiaprofenic 200mg tab Apo-Tiaprofenic 300mg tab Novo-Tiaprofenic 300mg tab Apo-Ticlopidine 250mg tab MYLAN-Ticlopidine 250mg tab Novo-Ticlopidine 250mg tab Sandoz Ticlopidine 250mg tab (discontinued) Ticlopidine 250mg tab AAP TEV TEV OMG CYI APX TEV APX TEV APX MYL TEV SDZ SAS 0.0713 0.1350 0.1817 1.1880 1.1880 terazosin 10mg tab testosterone undercanoate 40mg cap theophylline 200mg SR tab theophylline 300mg SR tab thiamine (vit B1) 100mg/mL inj tiaprofenic acid 200mg tab tiaprofenic acid 300mg tab ticlopidine 250mg tab (exception status) 0.2333 0.2333 0.3257 0.3257 0.4398 0.4398 0.4398 0.4398 0.4398 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 79 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength timolol maleate 0.25% oph gel DIN 02242275 02171880 Brand Timolol Maleate-EX 0.25% oph gel Timoptic-XE 0.25% oph gel MFR MRP SDZ 2.5920 FRS 2.5920 timolol maleate 0.5% oph gel 02242276 02171899 Timolol Maleate-EX 0.5% oph gel Timoptic-XE 0.5% oph gel SDZ FRS 2.7300 2.7300 timolol maleate 0.25% oph sol 00755826 00893773 02083353 02166712 Apo-Timop 0.25% oph sol MYLAN-Timolol 0.25% oph sol (discontinued) pms-Timolol 0.25% oph sol Sandoz Timolol 0.25% oph sol APX MYL PMS SDZ 0.9678 0.9678 0.9678 0.9678 timolol maleate 0.5% oph sol 00755834 00893781 02083345 02166720 00451207 Apo-Timop 0.5% oph sol MYLAN-Timolol 0.5% oph sol (discontinued) pms-Timolol 0.5% oph sol Sandoz Timolol 0.5% oph sol Timoptic 0.5% oph sol APX MYL PMS SDZ FRS 1.2754 1.2754 1.2754 1.2754 1.2754 timolol maleate 5mg tab 00755842 01947796 Apo-Timol 5mg tab Novo-Timol 5mg tab APX TEV 0.1649 0.1649 timolol maleate 10mg tab 00755850 01947818 00755869 01947826 02259893 02272059 02239170 02241209 Apo-Timol 10mg tab Novo-Timol 10mg tab Apo-Timol 20mg tab Novo-Timol 20mg tab Apo-Tizanidine 4mg tab MYLAN-Tizanidine 4mg tab Zanaflex 4mg tab Tobramycin 10mg/mL inj APX TEV APX TEV APX MYL SQI SDZ 0.2572 0.2572 0.5005 0.5005 0.3686 0.3686 0.3686 2.3150 02382814 02241210 02241755 00513962 Tobramycin 40mg/mL inj (AJP) Tobramycin 40mg/mL inj (SDZ) Sandoz Tobramycin 0.3% oph sol Tobrex 0.3% oph sol AJP SDZ SDZ ALC 3.2100 3.2100 0.5948 0.5948 00312762 02279614 02345803 02287765 02352850 02315645 02263351 02248860 02271184 02262991 02260050 02230893 02356856 02325136 02279630 02345838 02287773 Tolbutamide 500mg tab Apo-Topiramate 25mg tab Auro-Topiramate 25mg tab CO Topiramate 25mg tab GD-Topiramate 25mg tab MINT-Topiramate 25mg tab MYLAN-Topiramate 25mg tab Novo-Topiramate 25mg tab phl-Topiramate 25mg tab pms-Topiramate 25mg tab SandozTopiramate 25mg tab Topamax 25mg tab Topiramate 25mg tab Zym-Topiramate 25mg tab Apo-Topiramate 100mg tab Auro-Topiramate 100mg tab CO Topiramate 100mg tab AAP APX ARO COB GMD MNT MYL TEV PHL PMS SDZ JAN SAS ZYM APX ARO COB 0.1182 0.4379 0.4379 0.4379 0.4379 0.4379 0.4379 0.4379 0.4379 0.4379 0.4379 0.4379 0.4379 0.4379 0.8300 0.8300 0.8300 timolol maleate 20mg tab tizanidine 4mg tab (exception status) tobramycin 10mg/mL inj tobramycin 40mg/mL inj tobramycin 0.3% oph sol tolbutamide 500mg tab topiramate 25mg tab (exception status) topiramate 100mg tab (exception status) Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 80 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength topiramate 100mg tab (exception status) DIN 02352877 Brand GD-Topiramate 100mg tab MFR MRP GMD 0.8300 02315653 02263378 02248861 02271192 02263009 02260069 02230894 02356864 02325144 MINT-Topiramate 100mg tab MYLAN-Topiramate 100mg tab Novo-Topiramate 100mg tab phl-Topiramate 100mg tab pms-Topiramate 100mg tab Sandoz Topiramate 100mg tab Topamax 100mg tab Topiramate 100mg tab Zym-Topiramate 100mg tab MNT MYL TEV PHL PMS SDZ JAN SAS ZYM 0.8300 0.8300 0.8300 0.8300 0.8300 0.8300 0.8300 0.8300 0.8300 02279649 02345846 02287781 02352885 02315661 02263386 02248862 02271206 02263017 02267837 02230896 02356872 02325152 02147637 02236941 01937227 02144263 02348772 Apo-Topiramate 200mg tab Auro-Topiramate 200mg tab CO Topiramate 200mg tab GD-Topiramate 200mg tab MINT-Topiramate 200mg tab MYLAN-Topiramate 200mg tab Novo-Topiramate 200mg tab phl-Topiramate 200mg tab pms-Topiramate 200mg tab Sandoz Topiramate 200mg tab Topamax 200mg tab Topiramate 200mg tab Zym-Topiramate 200mg tab Apo-Trazodone 50mg tab phl-Trazodone 50mg tab pms-Trazodone 50mg tab Teva-Trazodone 50mg tab Trazodone 50mg tab APX ARO COB GMD MNT MYL TEV PHL PMS SDZ JAN SAS ZYM APX PHL PMS TEV SAS 1.2395 1.2395 1.2395 1.2395 1.2395 1.2395 1.2395 1.2395 1.2395 1.2395 1.2395 1.2395 1.2395 0.0775 0.0775 0.0775 0.0775 0.0775 trazodone 100mg tab 02147645 02236942 01937235 02144271 02348780 Apo-Trazodone 100mg tab phl-Trazodone 100mg tab pms-Trazodone 100mg tab Teva-Trazodone 100mg tab Trazodone 100mg tab APX PHL PMS TEV SAS 0.1385 0.1385 0.1385 0.1385 0.1385 trazodone 150mg tab 02147653 02144298 02348799 01964054 01999761 02229540 01999869 01977563 02229550 00345539 00312754 Apo-Trazodone 150mg tab Teva-Trazodone 150mg tab Trazodone 150mg tab Oracort 0.1% Paste Kenalog-10 10mg/mL inj Triamcinolone 10mg/mL inj Kenalog-40 40mg/mL inj Triamcinolone 40mg/mL inj Triamcinolone 40mg/mL inj Trifluoperazine 1mg tab Trifluoperazine 2mg tab APX TEV SAS TAR WSQ SDZ WSQ CYI SDZ AAP AAP 0.2035 0.2035 0.2035 0.9267 2.6760 2.6760 4.7700 4.7700 4.7700 0.1454 0.1908 topiramate 200mg tab (exception status) trazodone 50mg tab triamcinolone acetonide 0.1% oral paste triamcinolone 10mg/mL inj triamcinolone 40mg/mL inj trifluoperazine 1mg tab trifluoperazine 2mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 81 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength trifluoperazine 5mg tab DIN 00312746 Brand Trifluoperazine 5mg tab MFR MRP AAP 0.2526 trifluoperazine 10mg tab 00326836 Trifluoperazine 10mg tab AAP 0.3028 trimebutine 200mg tab 00803499 02245664 Modulon 200mg tab Trimebutine 200mg tab AXC AAP 0.5680 0.5680 trimethoprim 100mg tab 02243116 02243117 00740799 Trimethoprim 100mg tab Trimethoprim 200mg tab Trimipramine 12.5mg tab AAP AAP AAP 0.2785 0.5722 0.2339 00740802 Trimipramine FCT 25mg tab AAP 0.3012 trimipramine 50mg tab 00740810 Trimipramine 50mg tab AAP 0.5896 trimipramine 75mg cap 02070987 Trimipramine 75mg cap AAP 0.7936 trimipramine 100mg tab tryptophan 500mg tab (exception status) 00740829 02248538 02240333 02029456 Trimipramine 100mg tab Apo-Tryptophan 500mg tab ratio-Tryptophan 500mg tab Tryptan 500mg tab AAP APX TEV VLN 1.0061 0.3563 0.3563 0.3563 tryptophan 1g tab (exception status) 02248539 02237250 00654531 Apo-Tryptophan 1g tab ratio-Tryptophan 1g tab Tryptan 1g tab APX TEV VLN 0.7126 0.7126 0.7126 tryptophan 500mg cap (exception status) 02248540 02240334 00718149 02273497 02238984 02273500 02245894 Apo-Tryptophan 500mg cap ratio-Tryptophan 500mg cap Tryptan 500mg cap pms-Ursodiol C 250mg tab Urso 250mg tab pms-Ursodiol C 500mg tab Urso DS 500mg tab APX TEV VLN PMS AXC PMS AXC 0.3563 0.3563 0.3563 0.9895 0.9895 1.8769 1.8769 02320673 Stelara 45mg/0.5mL syringe inj JAN 02295822 02331748 02351579 02298457 02219492 02238048 00443840 02184648 02100630 02230768 02229628 Apo-Valacyclovir 500mg tab CO-Valacyclovir 500mg tab MYLAN-Valacyclovir 500mg tab pms-Valacyclovir 500mg tab Valtrex 500mg tab Apo-Valproic 250mg cap Depakene 250mg cap MYLAN-Valproic 250mg cap Novo-Valproic 250mg cap pms-Valproic 250mg cap pms-Valproic 500mg EC cap APX COB MYL PMS GSK APX ABB MYL TEV PMS PMS 1.1874 1.1874 1.1874 1.1874 1.1874 0.1947 0.1947 0.1947 0.1947 0.1947 0.5197 02238370 00443832 02236807 02140063 02371510 02337487 02270528 Apo-Valproic 50mg/mL syr Depakene 50mg/mL syr pms-Valproic 50mg/mL syr ratio-Valproic 50mg/mL syr Apo-Valsartan 40mg tab CO Valsartan 40mg tab Diovan 40mg tab APX ABB PMS TEV APX COB NVR 0.0406 0.0406 0.0406 0.0406 0.4075 0.4075 0.4075 trimethoprim 200mg tab trimipramine 12.5mg tab trimipramine 25mg tab ursodiol 250mg tab (exception status) ursodiol 500mg tab (exception status) ustekinumab 90mg/mL inj (exception status) valacyclovir 500mg tab valproic acid 250mg cap valproic acid 500mg EC cap valproic acid 50mg/mL syr valsartan 40mg tab PRP 9967.1138 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 82 of 87 NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength valsartan 40mg tab DIN 02383527 02312999 02363062 02356740 02356643 02366940 Brand MYLAN-Valsartan 40mg tab pms-Valsartan 40mg tab RAN-Valsartan 40mg tab Sandoz Valsartan 40mg tab Teva-Valsartan 40mg tab Valsartan 40mg tab MFR MRP MYL 0.4075 PMS 0.4075 RAN 0.4075 SDZ 0.4075 TEV 0.4075 SAS 0.4075 valsartan 80mg tab 02371529 02337495 02244781 02383535 02313006 02363100 02356759 02356651 02366959 Apo-Valsartan 80mg tab CO Valsartan 80mg tab Diovan 80mg tab MYLAN-Valsartan 80mg tab pms-Valsartan 80mg tab RAN-Valsartan 80mg tab Sandoz Valsartan 80mg tab Teva-Valsartan 80mg tab Valsartan 80mg tab APX COB NVR MYL PMS RAN SDZ TEV SAS 0.4188 0.4188 0.4188 0.4188 0.4188 0.4188 0.4188 0.4188 0.4188 valsartan 160mg tab 02371537 02337509 02244782 02383543 02313014 02363119 02356767 02356678 02366967 02371545 02337517 02289504 02383551 02344564 02356775 02356686 02366975 Apo-Valsartan 160mg tab CO Valsartan 160mg tab Diovan 160mg tab MYLAN-Valsartan 160mg tab pms-Valsartan 160mg tab RAN-Valsartan 160mg tab Sandoz Valsartan 160mg tab Teva-Valsartan 160mg tab Valsartan 160mg tab Apo-Valsartan 320mg tab CO Valsartan 320mg tab Diovan 320mg tab MYLAN-Valsartan 320mg tab pms-Valsartan 320mg tab Sandoz Valsartan 320mg tab Teva-Valsartan 320mg tab Valsartan 320mg tab APX COB NVR MYL PMS RAN SDZ TEV SAS APX COB NVR MYL PMS SDZ TEV SAS 0.4198 0.4198 0.4198 0.4198 0.4198 0.4198 0.4198 0.4198 0.4198 0.4080 0.4080 0.4080 0.4080 0.4080 0.4080 0.4080 0.4080 02382547 Apo-Valsartan/HCTZ 80/12.5mg tab APX 0.4176 02241900 02373734 02356694 02356996 02367009 02382555 Diovan-HCT 80/12.5mg tab MYLAN-Valsartan HCTZ 80/12.5mg tab Sandoz Valsartan/HCT 80/12.5mg tab Teva-Valsartan/HCTZ 80/12.5mg tab Valsartan HCT 80/12.5mg tab Apo-Valsartan/HCTZ 160/12.5mg tab NVR MYL SDZ TEV SAS APX 0.4176 0.4176 0.4176 0.4176 0.4176 0.4190 02241901 02373742 02356708 02357003 Diovan-HCT 160/12.5mg tab MYLAN-Valsartan HCTZ 160/12.5mg tab Sandoz Valsartan/HCT 160/12.5mg tab Teva-Valsartan/HCTZ 160/12.5mg tab NVR MYL SDZ TEV 0.4190 0.4190 0.4190 0.4190 valsartan 320mg tab valsartan 80mg & hydrochlorothiazide 12.5mg tab valsartan 160mg & hydrochlorothiazide 12.5mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 83 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength valsartan 160mg & hydrochlorothiazide 12.5mg tab valsartan 160mg & hydrochlorothiazide 25mg tab DIN 02367017 Brand Valsartan HCT 160/12.5mg tab MFR MRP SAS 0.4190 02382563 Apo-Valsartan/HCTZ 160/25mg tab APX 0.4179 02246955 02373750 02356716 02357011 02367025 Diovan-HCT 160/25mg tab MYLAN-Valsartan HCTZ 160/25mg tab Sandoz Valsartan/HCT 160/25mg tab Teva-Valsartan/HCTZ 160/25mg tab Valsartan HCT 160/25mg tab NVR MYL SDZ TEV SAS 0.4179 0.4179 0.4179 0.4179 0.4179 02382571 Apo-Valsartan/HCTZ 320/12.5mg tab APX 0.4204 02308908 02373769 02356724 02357038 02367033 02382598 Diovan-HCT 320/12.5mg tab MYLAN-Valsartan HCTZ 320/12.5mg tab Sandoz Valsartan/HCT 320/12.5mg tab Teva-Valsartan/HCTZ 320/12.5mg tab Valsartan HCT 320/12.5mg tab Apo-Valsartan/HCTZ 320/25mg tab NVR MYL SDZ TEV SAS APX 0.4204 0.4204 0.4204 0.4204 0.4204 0.4179 02308916 02373777 02356732 02357046 02367041 00800430 Diovan-HCT 320/25mg tab MYLAN-Valsartan HCTZ 320/25mg tab Sandoz Valsartan/HCT 320/25mg tab Teva-Valsartan/HCTZ 320/25mg tab Valsartan HCT 320/25mg tab Vancocin 125mg cap NVR MYL SDZ TEV SAS MRS 0.4179 0.4179 0.4179 0.4179 0.4179 6.1090 02377470 Vancomycin HCl 125mg cap PPC 6.1090 00788716 Vancocin 250mg cap MRS 12.2065 02377489 02230191 02342855 Vancomycin HCl 250mg cap Sterile Vancomycin HCI 500mg/vial inj Val-Vanco 500mg/vial inj PPC 12.2065 HOS 33.6893 VAL 33.6893 vancomycin HCI 1g/vial inj 02230192 02342863 Sterile Vancomycin HCI 1g/vial inj Val-Vanco 1000mg/vial inj HOS 64.0042 VAL 64.0042 vancomycin 500mg/vial inj vancomycin 1g/vial inj venlafaxine 37.5mg ER cap 02241820 pms-Vancomycin 500mg/vial inj PMS 33.6893 02241821 pms-Vancomycin 1g/vial inj PMS 64.0042 02331683 02304317 02237279 02360020 02310279 02278545 02380072 02273969 02310317 02275023 02354713 Apo-Venlafaxine 37.5mg XR cap CO Venlafaxine 37.5mg XR cap Effexor 37.5mg XR cap GD-Venlafaxine XR 37.5mg cap MYLAN-Venlafaxine 37.5mg XR cap pms-Venlafaxine 37.5mg XR cap RAN-Venlafaxine 37.5mg XR cap ratio-Venlafaxine 37.5mg XR cap Sandoz Venlafaxine 37.5mg XR cap Teva-Venlafaxine XR 37.5mg cap Venlafaxine 37.5mg XR cap APX COB WAY GMD MYL PMS RAN TEV SDZ TEV SAS valsartan 320mg & hydrochlorothiazide 12.5mg tab valsartan 320mg & hydrochlorothiazide 25mg tab vancomycin HCl 125mg cap (exception status) vancomycin HCl 250mg cap (exception status) vancomycin HCI 500mg/vial inj 0.1643 0.1643 0.1643 0.1643 0.1643 0.1643 0.1643 0.1643 0.1643 0.1643 0.1643 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 84 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength venlafaxine 75mg ER cap DIN 02331691 02304325 02237280 02360039 02310287 02275031 02278553 02380080 02273977 02310325 02354721 Brand Apo-Venlafaxine 75mg XR cap CO Venlafaxine 75mg XR cap Effexor 75mg XR cap GD-Venlafaxine XR 75mg cap MYLAN-Venlafaxine 75mg XR cap Novo-Venlafaxine 75mg XR cap pms-Venlafaxine 75mg XR cap RAN-Venlafaxine 75mg XR cap ratio-Venlafaxine 75mg XR cap Sandoz Venlafaxine 75mg XR cap Venlafaxine 75mg XR cap MFR MRP APX 0.3285 COB 0.3285 WAY 0.3285 GMD 0.3285 MYL 0.3285 TEV 0.3285 PMS 0.3285 RAN 0.3285 TEV 0.3285 SDZ 0.3285 SAS 0.3285 venlafaxine 150mg ER cap 02331705 02304333 02237282 02360047 02310295 02278561 02380099 02273985 02310333 02275058 02354748 00782483 02237921 00782491 02237922 Apo-Venlafaxine 150mg XR cap CO Venlafaxine 150mg XR cap Effexor 150mg XR cap GD-Venlafaxine XR 150mg cap MYLAN-Venlafaxine 150mg XR cap pms-Venlafaxine 150mg XR cap RAN-Venlafaxine 150mg XR cap ratio-Venlafaxine 150mg XR cap Sandoz Venlafaxine 150mg XR cap Teva-Venlafaxine XR 150mg cap Venlafaxine 150mg XR cap Apo-Verap 80mg tab MYLAN-Verapamil 80mg tab Apo-Verap 120mg tab MYLAN-Verapamil 120mg tab APX COB WAY GMD MYL PMS RAN TEV SDZ TEV SAS APX MYL APX MYL 0.3469 0.3469 0.3469 0.3469 0.3469 0.3469 0.3469 0.3469 0.3469 0.3469 0.3469 0.2735 0.2735 0.4250 0.4250 02246894 01934317 02210355 02246895 00742554 02210363 02211920 02237791 02242924 01918311 02244462 02265273 02242680 02344025 02242925 01918338 02244463 02265281 02242681 Apo-Verap 180mg SR tab Isoptin 180mg SR tab MYLAN-Verapamil 180mg SR tab Apo-Verap 240mg SR tab Isoptin 240mg SR tab MYLAN-Verapamil 240mg SR tab Novo-Veramil 240mg SR tab pms-Verapamil 240mg SR tab Apo-Warfarin 1mg tab Coumadin 1mg tab MYLAN-Warfarin 1mg tab Novo-Warfarin 1mg tab Taro-Warfarin 1mg tab Warfarin 1mg tab Apo-Warfarin 2mg tab Coumadin 2mg tab MYLAN-Warfarin 2mg tab Novo-Warfarin 2mg tab Taro-Warfarin 2mg tab APX ABB MYL APX ABB MYL TEV PMS APX BRI MYL TEV TAR SAS APX BRI MYL TEV TAR 0.5424 0.5424 0.5424 0.7233 0.7233 0.7233 0.7233 0.7233 0.1114 0.1114 0.1114 0.1114 0.1114 0.1114 0.1178 0.1178 0.1178 0.1178 0.1178 verapamil HCl 80mg tab verapamil HCl 120mg tab verapamil 180mg SR tab verapamil 240mg SR tab warfarin 1mg tab warfarin 2mg tab Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 85 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength warfarin 2mg tab DIN 02344033 Warfarin 2mg tab MFR MRP SAS 0.1178 warfarin 2.5mg tab 02242926 01918346 02244464 02265303 02242682 02344041 02245618 02240205 02287498 02265311 02242683 02344068 Apo-Warfarin 2.5mg tab Coumadin 2.5mg tab MYLAN-Warfarin 2.5mg tab Novo-Warfarin 2.5mg tab Taro-Warfarin 2.5mg tab Warfarin 2.5mg tab Apo-Warfarin 3mg tab Coumadin 3mg tab MYLAN-Warfarin 3mg tab Novo-Warfarin 3mg tab Taro-Warfarin 3mg tab Warfarin 3mg tab APX BRI MYL TEV TAR SAS APX BRI MYL TEV TAR SAS 0.0943 0.0943 0.0943 0.0943 0.0943 0.0943 0.1460 0.1460 0.1460 0.1460 0.1460 0.1460 warfarin 4mg tab 02242927 02007959 02244465 02265338 02242684 02344076 Apo-Warfarin 4mg tab Coumadin 4mg tab MYLAN-Warfarin 4mg tab Novo-Warfarin 4mg tab Taro-Warfarin 4mg tab Warfarin 4mg tab APX BRI MYL TEV TAR SAS 0.1460 0.1460 0.1460 0.1460 0.1460 0.1460 warfarin 5mg tab 02242928 01918354 02244466 02265346 02242685 02344084 Apo-Warfarin 5mg tab Coumadin 5mg tab MYLAN-Warfarin 5mg tab Novo-Warfarin 5mg tab Taro-Warfarin 5mg tab Warfarin 5mg tab APX BRI MYL TEV TAR SAS 0.0945 0.0945 0.0945 0.0945 0.0945 0.0945 warfarin 6mg tab 02240206 02287501 Coumadin 6mg tab MYLAN-Warfarin 6mg tab BRI MYL 0.1753 0.1753 warfarin 7.5mg tab 02287528 02242697 02242929 01918362 02244467 02242687 02344114 02369036 02324229 02362988 02313960 02238660 MYLAN-Warfarin 7.5mg tab Taro-Warfarin 7.5mg tab Apo-Warfarin 10mg tab Coumadin 10mg tab MYLAN-Warfarin 10mg tab Taro-Warfarin 10mg tab Warfarin 10mg tab MYLAN-Zolmitriptan 2.5mg tab pms-Zolmitriptan 2.5mg tab Sandoz Zolmitriptan 2.5mg tab Teva-Zolmitriptan 2.5mg tab Zomig 2.5mg tab MYL TAR APX BRI MYL TAR SAS MYL PMS SDZ TEV AZE 0.3014 0.3014 0.1695 0.1695 0.1695 0.1695 0.1695 4.8008 4.8008 4.8008 4.8008 4.8008 02387158 MYLAN-Zolmitriptan ODT 2.5mg tab MYL 4.8008 02324768 02362996 02342545 02243045 pms-Zolmitriptan ODT 2.5mg tab Sandoz Zolmitriptan ODT 2.5mg tab Teva-Zolmitriptan OD 2.5mg tab Zomig Rapimelt 2.5mg tab PMS SDZ TEV AZE 4.8008 4.8008 4.8008 4.8008 warfarin 3mg tab warfarin 10mg tab zolmitriptan 2.5mg tab (exception status) zolmitriptan ODT 2.5mg tab (exception status) Brand Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 86 of 87 PRP NOVA SCOTIA PHARMACARE PROGRAMS REIMBURSEMENT LIST - April 2013 2 1 Generic Name and Strength zopiclone 5mg tab DIN 02245077 02271931 02216167 02391716 02296616 02251450 02294052 02243426 02267918 02246534 02257572 02344122 Brand Apo-Zopiclone 5mg tab CO Zopiclone 5mg tab Imovane 5mg tab MINT-Zopiclone 5mg tab MYLAN-Zopiclone 5mg tab Novo-Zopiclone 5mg tab phl-Zopiclone 5mg tab pms-Zopiclone 5mg tab RAN-Zopiclone 5mg tab ratio-Zopiclone 5mg tab Sandoz Zopiclone 5mg tab Zopiclone 5mg tab MFR MRP APX 0.2231 COB 0.2231 SAV 0.2231 MNT 0.2231 MYL 0.2231 TEV 0.2231 PHL 0.2231 PMS 0.2231 RAN 0.2231 TEV 0.2231 SDZ 0.2231 SAS 0.2231 Key: 1. MRP = Maximum reimbursable price. The beneficiary is not to be charged any cost difference between the actual acquisition cost of the drug and the MRP. 2. PRP = Pharmacare reimbursement price. The beneficiary is always to be charged the cost difference between the actual acquisition cost of the drug and the PRP unless a PRP exception has been approved. Version: NS Pharmacare Reimbursement List Effective April 2013 Page 87 of 87 PRP MAY 2013 • VOLUME 13-03 PHARMACISTS’ EDITION Nova Scotia Formulary Updates Nova Scotia Formulary Updates Benefit Status Change: Clarithromycin and Azithromycin Benefit Status Change: Clarithromycin and Azithromycin Criteria Update Effective June 1, 2013, as a result of a recommendation of the Atlantic Expert Advisory Committee: - Triptans New Exception Status Benefits - Zenhale Trajenta New Products Clarithromycin will be listed as a regular benefit in the Nova Scotia Pharmacare Programs (no longer requiring a criteria code or special authorization request). Changes to the Nova Scotia Formulary Auditor’s Corner Clarithromycin is considered a first line alternative for community acquired pneumonia in a previously healthy, low risk adult patient with no risk factors for drug-resistant S. pneumonia (doxycycline is also a recommended first line alternative). Clarithromycin should be reserved as an alternative first line therapy for “simple” exacerbations of chronic bronchitis when other first line agents (including amoxicillin, doxycycline, or cefuroxime) are not appropriate due to its diminished activity versus H. influenza. Clarithromycin should not be routinely used for the first line treatment of uncomplicated upper respiratory tract infections such as pharyngitis and sinusitis because of limited evidence of superiority over the first line agents such as penicillin V and amoxicillin. Azithromycin will continue to be listed as a restricted benefit. Evidence suggests azithromycin may promote macrolide resistance to a greater extent than the use of clarithromycin, therefore coverage will be reserved for the following distinct treatment areas: The treatment of infections requiring a macrolide antibiotic when the patient has a documented intolerance to clarithromycin [Criteria Code 02] The treatment of chlamydia trachomatis as a single dose of 1g [Criteria Code 05] The treatment and prevention of mycobacterium avium complex (MAC) [Criteria Code 06] The treatment of infections requiring a macrolide antibiotic when the patient is taking medications that would significantly interact with erythromycin/clarithromycin [Criteria Code 07] PAGE 2 OF 6 PHARMACISTS’ EDITION VOLUME 13-03 Benefit Status Change: Clarithromycin and Azithromycin Continued… Decision Highlights The macrolides have an established place in therapy in lower respiratory tract infections, infections caused by Mycobacterium avium complex (MAC), infections caused by Helicobacter pylori and sexually transmitted diseases, including Chlamydia trachomatis. Compared to erythromycin, azithromycin and clarithromycin have improved kinetic and dynamic properties (bioavailability, tissue penetration), acid stability and tolerability, as well as a broader spectrum of activity. Azithromycin and clarithromycin also have potential limitations. Azithromycin has a longer half-life and remains in tissue at sub-inhibitory concentrations for extended periods of time which may promote macrolide resistance. Clarithromycin inhibits the CYPP3A4 enzyme system and has a number of potential drug interactions to consider. Health care providers should also be mindful of risk of prolonged QT interval and cardiac arrhythmia. Criteria Update – Triptans Please note that effective June 1, 2013, the criteria for all insured Selective 5HT1 Receptor Agonists will be updated to the following: Sumatriptan 50mg & 100mg Tablet, Naratriptan Tablet, Rizatriptan Tablet & Wafer, Zolmitriptan Tablet - for the treatment of migraine1 headache when: migraines are moderate2 in severity and other therapies (e.g. NSAIDs, acetaminophen, DHE spray) are not effective, or migraine attacks are severe2 or ultra severe2 - coverage limited to 18 doses/3 months3 patients with >3 migraines/month on average despite prophylactic therapy may be considered for up to a maximum of 12 doses/30days Almotriptan Tablet, Zolmitiptan Nasal Spray, Sumatriptan Nasal Spray - for the treatment of migraine1 headache of moderate2 intensity when: other therapies (e.g. NSAIDs, acetaminophen, DHE spray) are not effective AND patients have not responded to oral sumatriptan, zolmitriptan, rizatriptan and naratriptan. for the treatment of migraine1 headache of severe2 or ultra severe2 intensity when patients have not responded to oral sumatriptan, zolmitriptan, rizatriptan, and/or naratriptan. - coverage limited to 18 doses/3 months3 patients with >3 migraines/month on average despite prophylactic therapy may be considered for up to a maximum of 12 doses/30 days PAGE 3 OF 6 PHARMACISTS’ EDITION VOLUME 13-03 Criteria Update – Triptans Continued… Sumatriptan 6mg/Syringe Injection - for the treatment of migraine1 headache of moderate2 intensity when: other therapies (e.g. NSAIDs, acetaminophen, DHE spray) are not effective AND oral and nasal triptans are not appropriate. for the treatment of migraine1 headache of severe2 or ultra severe2 intensity when oral and nasal triptans are not appropriate. - coverage limited to 18 doses/3 months3 patients with >3 migraines/month on average despite prophylactic therapy may be considered for up to a maximum of 12 doses/30 days 1 As diagnosed based on current Canadian guidelines. Moderate – pain is distracting causing need to slow down and limit activities; Severe – pain affects ability to concentrate and very difficult to continue with daily activities; Ultra severe – unable to speak or think clearly; not able to function; likely lying down or sleeping. 3 Reimbursement will be available for a maximum quantity of 18 triptan doses per quarter (e.g., Jan to Mar) regardless of the agent(s) used within the 90 day period. 2 Definitions: New Exception Status Benefits The following products were reviewed by the Canadian Drug Expert Committee (CDEC) and will be listed as exception status benefits, with the following criteria, effective June 1, 2013. PRODUCT STRENGTH DIN PRESCRIBER BENEFIT STATUS MFR DNP 02361744 50/5mcg Inh FRS E(SF) 02361752 100/5mcg Inh 02361760 200/5mcg Inh Criteria for the treatment of moderate to severe asthma in patients who: are compliant with inhaled corticosteroids at optimal doses; and require additional symptom control, (e.g., cough, awakening at night, missing activities such as school, work or social activities because of asthma symptoms); and require increasing amounts of short-acting beta2-agonists, indicative of poor control Decision Highlights Zenhale is a fixed dose combination of an inhaled corticosteroid (mometasone furoate) and a long acting beta agonist (formoterol fumarate dehydrate) indicated for the maintenance treatment of asthma in adults and children 12 years of age and older who are not adequately controlled on asthma controller medications. Three randomized trials demonstrated that combination use of mometasone/formoterol was more efficacious than mometasone monotherapy for improving lung function in patients with asthma, as measured by FEV1. Zenhale is not indicated for the use in patients whose asthma can be successfully managed by inhaled corticosteroids along with occasional use of rapid onset, short duration, inhaled beta2-agonist Zenhale® (mometasone & formeterol) PAGE 4 OF 6 PHARMACISTS’ EDITION VOLUME 13-03 New Exception Status Benefits Continued… PRODUCT STRENGTH DIN PRESCRIBER Trajenta® (linagliptin) 5mg Tab 02370921 DNP BENEFIT STATUS E(SF) MFR BOE Criteria for the treatment of Type II diabetes for patients with: inadequate glycemic control on metformin and a sulfonylurea; and for whom insulin is not an option Decision Highlights Linagliptin is a selective DPP-4 inhibitor. The recommended dose is 5mg once daily. One RCT of patients with inadequate glycemic control on a combination of metformin and a sulfonylurea demonstrated a reduction of A1C with linagliptin therapy (MD-0.62%). A national CADTH Therapeutic Review Panel recommended that NPH insulin is the preferred next therapeutic option in patients who are not adequately controlled on metformin and a sulfonylurea. Linagliptin will only be insured in patients who are not able to use insulin. New Products The following products are new listings to the Nova Scotia Formulary, effective June 1, 2013. The benefit status within the Nova Scotia Pharmacare Programs is indicated. PRODUCT STRENGTH DIN PRESCRIBER Creon 6mg Minimicrospheres 80025653 DNP BENEFIT STATUS SF Tamiflu 6mg/mL Susp 02381842 HLR Mar-Atenolol 25mg Tab 02371979 DNP Not Insured SF Amlodipine-ODAN 2.5mg Tab 02378744 DNP SF ODN Allerject 0.15mg/0.15mL Inj 02382059 DNPM SF1 SAV Allerject 0.3mg/0.3mL Inj 02382067 DNPM SF1 SAV MFR ABB MAR Allerject has a quantity limit of two injections per fiscal year. The prescriber may submit a request to the Pharmacare office for consideration should beneficiaries require more than two injections per fiscal year. 1 PAGE 5 OF 6 PHARMACISTS’ EDITION VOLUME 13-03 Changes to the Nova Scotia Formulary Beginning in June 2013, the Nova Scotia Formulary will have a new look. It will continue to be updated on a monthly basis in a searchable, PDF file. Some of the new features are: Maximum Reimbursable Price (MRP)/Pharmacare Reimbursement Price (PRP) will be indicated and will replace the current reimbursement list Manufacturer List Prices (MLP) will be indicated for all non-interchangeable benefits Interchangeability will be indicated by a Y or an N Available in an Excel file for system uploads PAGE 6 OF 6 PHARMACISTS’ EDITION VOLUME 13-03 Auditors Corner – Pharmacy Closing or Transferring Ownership As indicated in the Tariff Agreement between the Pharmacy Association of Nova Scotia and the Nova Scotia Department of Health and Wellness, if your pharmacy is closing or changing ownership, it is your responsibility to notify our office within 30 days in advance of transfer/closing. This information will be retained in confidence, but a close-out prescription audit is required. New providers and providers who have changed ownership are required to complete the following forms provided by Pharmacare: Registration of the Pharmacy form, providing information to establish the pharmacy as an authorized provider of pharmaceutical services under the Pharmacare Programs. Confirmation of Agreement form, as acceptance of the Tariff Agreement. MSI Provider Business Arrangement form, authorizing direct payment to the pharmacy’s account. Provider Accreditation Application form, to request accreditation of the pharmacy’s software package and to accept the Terms and Conditions of MSI Provider Accreditation. Certification of Responsibility for Electronic Claims Submission form, to accept legal responsible and liability for the accuracy and validity of all claims submitted to Medavie Blue Cross via telecommunications. For more information, or to advise of a change you may contact our office using one of the following: E-mail: msiproviders@medavie.bluecross.ca Phone: (902) 496-7560, 496-7190, and 496-7107 Toll-free: 1-866-553-0585 Fax: 1-877-910-4674 JULY 2013 • VOLUME 13-04 PHARMACISTS’ EDITION Nova Scotia Formulary Updates Nova Scotia Formulary Updates Benefit Status Change: Proton Pump Inhibitors Benefit Status Change: Proton Pump Inhibitors New Exception Status Benefits As a result of a recent utilization review of proton pump inhibitors (PPIs) and differences in costs within this category, the benefit status of PPIs is being reviewed. It should be noted that studies indicate that there are no clinically important differences among standard doses of PPIs. As an initial stage: - Gilenya® Invega Sustenna® Onbrez® Non-Insured Products - Lodalis® Fampyra® Latuda® New Diabetic Product Exception Status Criteria Reminder Prescriber Validation Prescriber License Numbers Palliative Home Care Drug Coverage Program Update Calcitonin Intranasal Criteria Code Auditor’s Corner • Effective September 1, 2013, Tecta® 40mg (pantoprazole magnesium) will be moved from a regular benefit to an exception status benefit (requiring special authorization). Patients currently using Tecta® will be grandfathered for coverage, pending additional analysis of PPI prescribing, utilization and costs. Please note that using higher than regular dosing of PPIs should rarely be required as regular dosing provides potent acid suppression and similar healing rates. In the exceptional instance where higher doses are required (e.g. double dosing), using the lowest cost PPI (rabeprazole 20mg) is encouraged. To this end, and to reduce the administrative burden for practitioners and patients, effective August 1, 2013: • • The maximum yearly quantity limit for rabeprazole (currently 425 tablets) will be removed. Going forward, patients requiring double dosing of a PPI will be expected to use rabeprazole first. The current quantity limit for other PPIs will remain unchanged (maximum 425 caps/tab per year). Please see Page 2 for full coverage information. Prescribers are reminded that, for uncomplicated acid-peptic disease, the lowest amount of acid suppression for the shortest length of time should be used and the need for acid suppressive therapy should be regularly reassessed. PAGE 2 OF 7 PHARMACISTS’ EDITION VOLUME 13-04 Comparative Costs and Benefit Status of PPIs (effective September 1, 2013) DRUG BENEFIT STATUS REIMBURSEMENT LEVELS Rabeprazole 10mg Open $0.1204 Rabeprazole 20mg Open $0.2408 Omeprazole 10mg Open – at standard dose (up to 425 tabs/caps per year) Open – at standard dose (up to 425 tabs/caps per year) $0.2059* Pantoprazole magnesium 40mg (Tecta®) Pantoprazole sodium 20mg Exception $0.7500 Exception $0.3538* Pantoprazole sodium 40mg Exception $0.7076 Lansoprazole 15mg Exception $0.3500* Lansoprazole 30mg Exception $0.7000 Esomeprazole 20mg Not Insured $1.8690 Esomeprazole 40mg Not Insured $1.8690 Omeprazole 20mg $0.4117 *Reimbursement level of omeprazole 10mg, pantoprazole 20mg and lansoprazole 15mg are based on 50% of the cost of the standard dose. The manufacturer list prices are $0.8167, $1.2750 and $0.7000 respectively, therefore patients would be responsible to pay the difference for these strengths. Funding Criteria for Insured PPIs Rabeprazole: Full benefit, no special authorization required Omeprazole: Standard dose: full benefit at usual daily dose (e.g. 20mg per day). Maximum 425 tabs/caps per year. Double dose: requires special authorization and must have failed standard daily doses of both omeprazole and rabeprazole. Coverage duration: 8 week trial, followed by up to one year of coverage. Use beyond the 8 week trial will be considered if step down to standard dose is not successful. Pantoprazole Sodium, Pantoprazole Magnesium and Lansoprazole: Standard dose: failure of a trial of all open benefit PPIs (omeprazole, rabeprazole). Maximum 425 tabs/caps per year. Double dose: failure of standard dose of requested agent and double doses of rabeprazole. Coverage duration: 8 week trial, followed by up to one year of coverage. Use beyond an 8 week trial will be considered if step down to standard dose is not successful. Note that concerns have been raised regarding a potential increased risk of clostridium difficile, hip fractures, iron and B12 deficiency and gastric polyps associated with PPI use. Therapy should regularly reassessed. Consider the role of lifestyle adjustments and the use of OTC products (e.g. alginates, antacids and H2 blockers) for appropriate patients. PAGE 3 OF 7 PHARMACISTS’ EDITION VOLUME 13-04 New Exception Status Benefits The following products have been reviewed by the Canadian Drug Expert Committee (CDEC) and will be listed as exception status benefits, with the following criteria effective August 1, 2013. PRODUCT DIN PRESCRIBER PRP BENEFIT STATUS E MFR Gilenya® 0.5mg Cap 02365480 DNP 93.4888 NVR (fingolimod) Criteria for the treatment of patients with relapsing remitting multiple sclerosis (RRMS) who meet all of the following criteria: • failure to respond to full and adequate courses* of at least one interferon or glatiramer acetate or documented intolerance** to both therapies. • one or more clinically disabling relapses in the previous year. • significant increase in T2 lesion load compared with that from a previous MRI scan (i.e. 3 or more new lesions) or at least one gadolinium-enhancing lesion. • requested and followed by a neurologist experienced in the management of RRMS • recent expanded disability status scale (EDSS) score of 5.5 or less (i.e. patients must be able to ambulate at least 100 meters without assistance). Dosage: 0.5mg daily Approval period: 1 year Exclusions: • not funded in combination with other disease modifying therapies • not funded in patients with an EDSS>5.5 • not funded in patients who have had a heart attack or stroke in the last six months of funding request, patients with a history of sick sinus syndrome, atrioventricular block, significant QT prolongations, bradycardia, ischemic heart disease, or congestive heart failure • not funded in patients <18 years of age • not funded due to needle phobia or preference for oral therapy over injection in patients without clinical contraindications to interferon or glatiramer therapy • Note: skin reactions at the site of injection do not qualify as contraindications to interferon or glatiramer therapy Renewal: • EDSS score ≤ 5.5 (i.e. patients must be able to ambulate at least 100 meters without assistance). Date and details of the most recent neurological examination and EDSS scores must be provided (exam must have occurred within that last 90 days) AND • Patients must be stable or have experienced no more than 1 disabling attack/relapse in the past year Of Note: *Failure to respond to full and adequate courses: defined as a trial of at least 6 months of interferon or glatiramer therapy AND experienced at least one disabling relapse (attack) while on interferon or glatiramer therapy **Intolerance is defined as: documented serious adverse effects or contraindications that are incompatible with further use of that class of drug PAGE 4 OF 7 PHARMACISTS’ EDITION VOLUME 13-04 New Exception Status Benefits Continued… PRODUCT STRENGTH DIN PRESCRIBER Invega Sustenna® (paliperidone) BENEFIT STATUS E MFR 50mg/0.5mL Inj 02354217 DNP JAN 75mg/0.75mL Inj 02354225 100mg/mL Inj 02354233 150mg/1.5mL Inj 02354241 Criteria • for patients having problems with compliance on an oral antipsychotic or • for patients who are currently receiving a conventional depot antipsychotic and are experiencing significant side effects (EPS or TD) or lack of efficacy Decision Highlights • Paliperidone is depot injection (injected once monthly) indicated for the treatment of schizophrenia. Paliperidone is a pro-drug of risperidone. PRODUCT STRENGTH Onbrez® (indacaterol) DIN PRESCRIBER BENEFIT STATUS E MFR 75mcg 02376938 DNP NVR Micronized powder for inhalation Criteria for the treatment of chronic obstructive pulmonary disease (COPD), if symptoms persist after 2-3 months of short-acting bronchodilator therapy (i.e., salbutamol at a maximum dose of 8 puffs/day or ipratropium at maximum dose of 12 puffs/day) • coverage can be provided without a trial of short-acting agent if: - there is spirometric evidence of at least moderate to severe airflow obstruction, (i.e., postbronchodilator values FEV1 < 60% and FEV1/FVC ratio < 0.7), and significant symptoms (i.e., MRC score of 3-5*) • combination therapy with tiotropium and a long-acting beta2 agonist/inhaled corticosteroid will only be considered if: - there is spirometric evidence of at least moderate to severe airflow obstruction (postbronchodilator values FEV1 < 60% and FEV1/FVC ratio < 0.7), and significant symptoms (i.e., MRC score of 3-5*) and - there is evidence of one or more moderate-to-severe exacerbations per year, on average, for 2 consecutive years requiring antibiotics and/or systemic (oral or intravenous) corticosteroids NOTE: Coverage of combination therapy with tiotropium and a long-acting beta2 agonist (without an inhaled corticosteroid) will not be considered due to insufficient evidence to support substantial benefit. If spirometry cannot be obtained, reasons must be clearly explained and other evidence regarding severity of condition must be provided for consideration (i.e., MRC scale). Spirometry reports from any point in time will be accepted. *Canadian Thoracic Society COPD Classification By Symptom/Disability: MRC= Medical Research Council Dyspnea Scale Decision Highlights • In two 12 week studies comparing indacaterol with placebo, indacaterol was associated with improvements in trough FEV1 (mean difference 0.12L and 0.14L) PAGE 5 OF 7 PHARMACISTS’ EDITION VOLUME 13-04 Non-Insured Products The following products were reviewed by the Canadian Drug Expert Committee (CDEC) and were not recommended to be listed as insured benefits within the Nova Scotia Pharmacare Programs. PRODUCT STRENGTH DIN PRESCRIBER BENEFIT STATUS Not Insured MFR Lodalis® 625mg Tablet 02373955 VLN (colesevelam) Decision Highlights • Colesevelam is more costly than other bile acid sequestrants and there are no randomized controlled trials directly comparing colesevelam with other bile acid sequestrants. PRODUCT STRENGTH DIN PRESCRIBER BENEFIT STATUS Not Insured MFR Fampyra® 10mg SR Tablet 02379910 BIG (fampridine) Decision Highlights • In two phase three trials, fampridine treated groups reported statistically significant improvements in walking speed, however no between-treatment differences in quality of life was reported and the estimated cost per quality adjusted life year (QALY) is high. PRODUCT STRENGTH DIN PRESCRIBER Latuda® (lurasidone) BENEFIT STATUS Not Insured MFR 40mgTablet 02387751 SUN 80mg Tablet 02387778 120mg Tablet 02387786 Decision Highlights • There is insufficient evidence from randomized controlled trials to establish the comparative efficacy of lurasidone relative to other less costly antipsychotics for acute treatment of schizophrenia. Delisted Products The Atlantic Expert Advisory Committee recommended Bezalip be delisted from the Atlantic Provincial Formularies. Effective June 15, 2013, Bezalip was delisted as a benefit under the Nova Scotia Pharmacare Programs. PRODUCT STRENGTH DIN PRESCRIBER Bezalip (bezafibrate) 400mg SR tab 02083523 DNP Decision Highlights • BENEFIT STATUS Delisted MFR TRB Bezafibrate is more costly than any other fiber acid derivative or statin and does not offer any significant therapeutic advantages in clinical or safety outcomes compared to other fibrates. PAGE 6 OF 7 PHARMACISTS’ EDITION VOLUME 13-04 New Diabetic Product The following product is a new listing to the Nova Scotia Formulary, effective August 1, 2013. The benefit status within the Nova Scotia Pharmacare Programs is indicated. PRODUCT DIN/PIN BD Ultra-Fine Syringes, 0.3cc, 31g 97799425 PRODUCT NUMBER 324919 PRESCRIBER DNP BENEFIT STATUS SFD MFR BTD Exception Status Criteria Reminder The Nova Scotia Pharmacare Programs no longer provides printed booklets containing current exception status criteria. Any booklets still remaining should be disposed of. The current criteria can be found on the Pharmacare website, www.nspharmacare.ca, under Exception Status Drugs section and can also be found under Appendix III in the NS Formulary. Prescriber Validation As previously advised, effective September 23, 2013, all pharmacists who prescribe (including continued care prescriptions) for Nova Scotia Pharmacare clients must be registered with Medavie Blue Cross in order to submit these claims. After registering, pharmacists will use their NSCP license number as their prescriber identification instead of 71111. Registration forms are available at www.medavie.bluecross.ca\healthprofessionals. Should you have any questions please contact Medavie Customer Service Centre 1-800-667-4511. When system changes take effect in September, the prescriber for each prescription will be validated based on provincial license number, province and provider type. As a result, the alternate prescriber numbers that had been assigned for some physicians will no longer be required. Effective September 23, 2013, please use the physician’s CPSNS licence number in lieu of these numbers. Provider number 9999 should only be used when the provider number cannot be located. Prescriber License Numbers Prescriber license numbers are readily available online from respective licensing authorities. Therefore, prescriber lists for physicians, nurse practitioners and optometrists will no longer be provided as bulletin attachments. If needed, the following websites can be consulted: http://www.cpsns.ns.ca/ (College of Physicians and Surgeons-Physician Search) http://www.crnns.ca (Nurse Practitioner- Licensing Roster) http://www.nsco.ca/ (Registered Optometrists) PAGE 7 OF 7 PHARMACISTS’ EDITION VOLUME 13-04 Palliative Home Care Drug Coverage Program Update In the February 2012 Pharmacare News Bulletin, details of the Palliative Home Care Drug Coverage Program were provided. This program was effective February 1, 2012 and is intended for use in end-of-life care at home. We are currently in the process of evaluating the program and as part of this are moving toward an online claims submission process. This will streamline the process for all involved and reduce the effort required to process these claims. We are working toward an implementation in late 2013, but will provide an update once it has been confirmed. Some reminders about the program: • • • • A valid prescription is required for medications that can be purchased over the counter. Hand written or cash register receipts will not be accepted. An official prescription receipt or report is required for reimbursement. Faxes or photocopies will not be accepted. The program does not provide coverage for any supplies or medical equipment, such a diabetic, wound or ostomy supplies Medication Authorization Forms are valid for six months from the effective date. Claims submitted with forms that are greater than six months old are not eligible. Please contact Pharmaceutical Services at 902-424-1596 with any questions regarding the program. Calcitonin Intranasal Criteria Code Please remember; Criteria Code 90 is available for use for calcitonin intranasal, for the following criteria only: • for the treatment of pain associated with osteoporotic fragility fractures, bone metastases or pathological fractures (short term up to 3 months) The code is limited for use to a maximum of 3 months, once per year. The prescriber may submit a request to the Pharmacare office for consideration for beneficiaries who require therapy beyond 3 months. Auditor’s Corner REMINDER In order to avoid an audit recovery for Pharmacist Prescribing, Continued Care Prescriptions (CCP) and AMRS/BMRS the following documentation is required: • Pharmacist Prescribing - the pharmacist must sign or initial the prescription as the prescriber thereby assuming full responsibility for the prescription. • Continued Care Prescription- must include either the notification sent to the physician or the notation “CCP” on the hard copy. Also, the CCP must be signed or initialled by the pharmacist thereby assuming full responsibility for the prescription. • BMRS - a consent form signed by both the pharmacist and patient, and a comprehensive drug review list also signed by both the pharmacist and the patient, must be available for review during the onsite audit. • AMRS - a consent form signed by both pharmacist and patient and all pertinent documentation must be available for review during the onsite audit. SEPTEMBER 2013 • VOLUME 13-05 PHARMACISTS’ EDITION Nova Scotia Formulary Updates Nova Scotia Formulary Updates Administration of Publicly Funded Influenza Vaccinations by Pharmacists Administration of Publicly Funded Influenza Vaccinations by Pharmacists Palliative Home Care Drug Coverage Program – Electronic Adjudication New Exception Status Benefits Pregabalin & Gabapentin New Benefit Tamsulosin CR Criteria Update Incivek and Victrelis Compounded Methadone Products Using Methadose™ Included With This Bulletin PHCDC Program Covered Drugs The new Pharmacy Act and associated regulations (including the Registration, Licensing and Professional Accountability Regulations and the Pharmacy Practice Regulations) were approved by the provincial government and came into effect August 6, 2013. With the development and approval of the standards of practice by the Nova Scotia College of Pharmacists (NSCP), pharmacists now have the authority to administer drugs by injection provided they meet the required training and application expectations set out by the Regulations and Standards of Practice. Obtaining Publicly Funded Influenza Vaccine Note: Only those pharmacies who have been in contact with PANS and assigned a quantity of vaccine from the dedicated supply will receive publicly funded influenza vaccine. A dedicated supply of publicly funded influenza vaccine, multi dose vials of Fluviral, is available for participating pharmacies to order at no charge from one of two wholesalers. Lawtons and Sobeys Pharmacies will receive publicly funded influenza vaccine from their company’s wholesale division, while all other participating pharmacies will receive it from McKesson. Note: Procedures for accessing additional vaccine, if available, will be communicated later in the influenza season. Both Fluviral and Agriflu may be available later in the influenza season. Wholesalers will not accept returns of vaccine. Unused vaccine may be transferred to other pharmacies if needed. Please retain outdated or damaged vaccine and a process for return will be communicated later in the influenza season. PAGE 2 OF 6 PHARMACISTS’ EDITION VOLUME 13-05 Administration of Publicly Funded Influenza Vaccinations by Pharmacists Continued… Lawtons and Sobeys Wholesale The publicly funded supply of vaccine will be available to participating pharmacies starting October 9, 2013. Lawtons and Sobeys stores will receive an automatic distribution of the vaccine on October 9th. Quantities will be communicated prior to the distribution. McKesson The publicly funded supply of vaccine will be available to participating pharmacies starting October 9, 2013. Pharmacies do not need to create a McKesson order for vaccine; it will be processed automatically by McKesson. The automatic order will only be for the quantity allocated for your pharmacy confirmed by PANS. McKesson is setting up a prebooking event where orders will be entered automatically based on the per store allocation confirmed by PANS. The order will arrive between October 9th and 11th with your regular McKesson order. Billing for Vaccine Administration During the 2013/2014 influenza season participating pharmacies will be able to access publicly funded influenza vaccine supply. A fee of $11.50 will be paid for the administration of the vaccine, as per the agreement between the Department of Health and Wellness and the Pharmacy Association of Nova Scotia (PANS). The fee can be billed to the Pharmacare Programs electronically beginning October 15, 2013 in the following manner: All residents of NS are eligible to have their influenza vaccinations administered by their pharmacists and billed to Pharmacare provided they have a valid Nova Scotia health card. Patients who do not have a health card should contact their local Public Health office for information on immunization. Residents with valid Nova Scotia health cards receive influenza vaccine free of charge. There are no copayments or deductibles associated with the vaccine. In order to bill Pharmacare in the patient’s insurance field, use the Nova Scotia Health card number as the patient ID and a carrier ID of NS. If the patient is already set up in your system with Pharmacare coverage (e.g., Seniors’ Pharmacare, Family Pharmacare) a separate patient file does not have to be set up. Claims should be submitted using the DIN of the vaccine administered to the patient, unless the patient is pregnant, or is a child receiving a second vaccine dose. In these instances the following PINS are to be used: Pregnant Women – Agriflu® PIN 93899922 – Fluviral® PIN 93899921 PAGE 3 OF 6 PHARMACISTS’ EDITION VOLUME 13-05 Administration of Publicly Funded Influenza Vaccinations by Pharmacists Continued… Second Dose for Children – Agriflu® PIN 93899920 – Fluviral® PIN 93899919 Participating pharmacies will be able to access the publicly funded vaccines at no charge, so claims should be submitted with the administration fee in the dispensing fee field. Pharmacies will not be reimbursed for ingredient costs or markups for these claims. Public Health Resources for Influenza Season Materials used by Public Health to promote influenza vaccination and educate the public, along with the Respiratory Watch Report are available on their website at: www.novascotia.ca/hpp/cdpc/resources/respiratory-diseases.asp Palliative Home Care Drug Coverage Program – Electronic Adjudication The Palliative Home Care Drug Coverage Program was developed to support end of life care at home and began with a manual claims submission process in February 2012. Effective October 15, 2013 the program will move to an online claims submission process. This will streamline the process for all involved and reduce the effort required to process these claims. Eligibility Eligibility of the program will not change. The Palliative Care team in each DHA will determine individual eligibility and will forward completed Medication Authorization forms to the Pharmacare office. The pharmacy will submit the claims with the Nova Scotia health card number as the patient identification number and a carrier ID of NS. Note: It may take up to two business days to have system eligibility set up for new clients in the program. If a new client’s eligibility is not in the system, claims may reject with the message “CLIENT ID ERROR. If you are having claims rejected and you have the patient’s Medication Authorization Form you can fax it to 902-4947423 or 1-855-640-7423. Coverage Renewal Patients are eligible under the program for six months from the date on the Medication Authorization Form. Renewal of coverage is completed by the Palliative Care team in the DHA and forwarded to the Pharmacare office to extend coverage. Other Coverage Patients are eligible for the program if they are enrolled in another Pharmacare Program. The adjudication system will automatically coordinate amongst the Pharmacare plans as claims are submitted using the patient’s Nova Scotia health card number. Patients are eligible for the program if they have private insurance. The program is the payer of last resort. All claims are to be submitted to private insurance first, before being submitted to the program. Cost Share There are no copayments, deductibles or premiums associated with the program. PAGE 4 OF 6 PHARMACISTS’ EDITION VOLUME 13-05 Palliative Home Care Drug Coverage Program – Electronic Adjudication Continued… Pricing All claims will be subject to the Tariff Agreement between The Department of Health and Wellness and the Pharmacy Association of Nova Scotia. Claims should be submitted following Pharmacare pricing policies as set out in the Pharmacists’ Guide and Pharmacare News Bulletins. Claims Incurred Prior to October 15, 2013 Any claims incurred prior to October 15 should continue to be manually submitted to the Department of Health and Wellness for payment. Pharmacies must submit a copy of the Medication Authorization form, along with the original prescription receipts, within six months of the date of service, to: Palliative Home Care Drug Coverage Program c/o Pharmaceutical Services, Department of Health and Wellness 1894 Barrington Street, PO Box 488 Halifax, NS B3J 2R8 Eligible Drugs All drugs eligible under the program will be regular benefits and do not require prior authorization. Please see enclosed reference chart for a list of insured medication categories. Contact Information Any questions regarding the program should be directed to Palliative Home Care Drug Coverage Program at 496-5680 or 1-800-305-5026. PAGE 5 OF 6 PHARMACISTS’ EDITION VOLUME 13-05 New Exception Status Benefits – Pregabalin & Gabapentin The Atlantic Expert Advisory Committee has reviewed the evidence for the use of pregabalin and gabapentin in the treatment of neuropathic pain and has recommended that both products be listed as exception status benefits effective October 15, 2013: Criteria: For the treatment of neuropathic pain (e.g. diabetic neuropathy, postherpetic neuropathy) in patients who have failed a trial of a tricylic antidepressant (e.g. amitriptyline, desipramine, imipramine, nortriptyline). Note: Patients who are already stabilized on gabapentin therapy have been grandfathered for coverage and, in addition, have also been approved for coverage for pregabalin. The following pregabalin categories are insured at the indicated MRPs. Please refer to the NS Formulary (October 2013) for the specific products insured. MRP DNP BENEFIT STATUS E pregabalin 50mg cap DNP E 0.4387 pregabalin 75mg cap DNP E 0.5676 pregabalin 150mg cap DNP E 0.8059 pregabalin 225mg cap DNP E 0.8059 pregabalin 300mg cap DNP E 0.8059 PRODUCT PRESCRIBER pregabalin 25mg cap (NOVEMBER 5, 2013) 0.2881 New Benefit – Tamsulosin CR Based on a review of the Atlantic Expert Advisory Committee, effective October 1, 2013, tamsulosin 0.4mg CR will become a full benefit in the Pharmacare Programs with the indicated MRP. BENEFIT STATUS MFR DNP SF APX 0.2168 DNP SF SDZ 02340208 0.2168 DNP SF SDZ 02270102 0.2168 DNP SF BOE MRP PRODUCT DIN tamsulosin 0.4mg sustained release cap/tab Apo-Tamsulosin 0.4mg CR Tab 02362406 0.2168 02295121 Sandoz Tamsulosin 0.4mg CR Cap Sandoz Tamsulosin 0.4mg CR Tab Flomax 0.4mg CR Tab (OCTOBER 22, 2013) PRESCRIBER PAGE 6 OF 6 PHARMACISTS’ EDITION VOLUME 13-05 Criteria Update – boceprevir (Incivek) and telaprevir (Victrelis) The Canadian Drug Expert Committee has recently reviewed their recommendation for funding of boceprevir and telaprevir for the treatment of chronic hepatitis C virus (HCV). Based on this review, effective October 1, 2013, the coverage criteria will be adjusted to remove the reference to HIV status, allowing for coverage in patients who are coinfected with HCV/HIV when other coverage criteria are met. Compounded Methadone products using Methadose™ Pharmacists are advised that they may choose to use Methadose™ (methadone) Oral Concentrate USP 10mg/ml solution (02394618) to prepare compounded methadone solutions that are billed to the Pharmacare Programs. All usual standards of practice and billing procedures apply. Regardless of whether a 10mg/mL stock solution compounded from methadone powder or a commercially prepared 10mg/mL methadone solutions is used in preparing the individual patient dose, the final product (individual patient dose q.s. to 100mL with Tang) is billed using the PIN 00999734 which is payable at an MLP of $0.0050/mg plus 10.5%. PHARMACISTS’ EDITION VOLUME 13-05 Nova Scotia Palliative Home Care Drug Coverage Program Insured Medications Analgesics Opioid analgesics NSAID Acetaminophen Dermatological Agents Corticosteroids Antifungals Antibiotics Respiratory Agents Cough preparations Bronchodilators Antihistamines Inhaled corticosteroids Inhaled anticholinergics (ipratropium, tiotropium) Anti-infective Agents (for dermatologic and systemic use) Antibiotics Antivirals (acyclovir, famciclovir, valacyclovir) Antifungals (clotrimazole, miconazole, terconazole, fluconazole, nystatin, ketoconazole) Diabetes Agents Insulin Gliclazide Metformin Rosiglitazone Glyburide Cardiovascular Agents Antiarrythmics (flecainide, mexiletene) Nitrates Beta blockers Calcium channel blockers Diuretics ACE inhibitors ARBs Bone Metabolism Regulators Bisphosphonates (pamidronate, clodronate, zoledronic acid) CNS Agents Anticonvulsants (gabapentin,pregabalin, carbamazepine, lamotrigine, phenytoin, phenobarbital, topiramate, valproic acid) Antidepressants Antipsychotics Stimulants (methylphenidate, modafinil, dextroamphetamine) Benzodiazpines Sedatives Anticoagulants Agents Warfarin Heparin LMWHs Miscellaneous Hemorrhoidal Agents Systemic corticosteroids Iron, folic acid, magnesium Gastrointestinal Agents Antidiarrheals Antiemetics (dimenhydrinate, prochlorperazine, domperidone, metoclopramide, promethazine, ondansetron, granisetron, dolasetron, nabilone, octreotide) Antispasmodics (atropine, glycopyrrolate, hyoscyamine, scopolamine) Laxatives PPIs H2 antagonists NOVEMBER 2013 • VOLUME 13-06 PHARMACISTS’ EDITION Nova Scotia Formulary Updates Nova Scotia Formulary Updates Administration of Publicly Funded Influenza Vaccinations by Pharmacists Administration of Publicly Funded Influenza Vaccinations by Pharmacists New Exception Status Benefits - Eliquis Xalkori Criteria Update - Xarelto Sutent New Diabetic Products Non-Insured Products - Apprilon Stribild Votrient Auditors Corner By now, all participating pharmacies have received their initial supply of publicly funded influenza vaccine and information from their local Public Health office on how to request additional supply. Pharmacies are reminded of the following key points: • • • • • Additional supply may include pre-filled syringes of Agriflu, in addition to the multi-dose vials of Fluviral. Public Health offices deal with many providers and must consider all of their requirements equally. Every attempt will be made to satisfy all requests, but there is no guarantee any provider will get the total amount of publicly funded vaccine requested. Please allow time for your request to be considered. Same day responses cannot be guaranteed and influenza clinics should not be planned without confirming supply is available. The process for each Public Health office varies. In order to minimize delays in processing requests, all providers who access publicly funded influenza vaccine must ensure any necessary forms are completed fully and all processes are followed (e.g., delivery and pick up of vaccine, cold chain requirements). All providers are responsible for any transportation or distribution costs to obtain additional vaccine supply. Billing for Vaccine Administration During the 2013/2014 influenza season participating pharmacies will be able to access publicly funded influenza vaccine supply. A fee of $11.50 will be paid for the administration of the vaccine, as per the agreement between the Department of Health and Wellness and the Pharmacy Association of Nova Scotia (PANS). All Nova Scotians receive influenza vaccine free of charge. There are no copayments or deductibles associated with the vaccine. PAGE 2 OF 6 PHARMACISTS’ EDITION VOLUME 13-06 Administration of Publicly Funded Influenza Vaccinations by Pharmacists Continued… A new billing process has been developed to ensure all Nova Scotians have more ways to get the influenza vaccine this year to help protect themselves and others from influenza. Now all Nova Scotians are eligible to have their influenza vaccinations administered by their pharmacists and billed to Pharmacare, regardless of whether or not they have a valid Nova Scotia health card. The fee can be billed to the Pharmacare Programs electronically in the following manner: • In order to bill Pharmacare in the patient’s insurance field, use the Nova Scotia Health card number as the patient ID and a carrier ID of NS. If the patient is already set up in your system with Pharmacare coverage (e.g., Seniors’ Pharmacare, Family Pharmacare) a separate patient file does not have to be set up. • Effective November 26, 2013 pharmacies may immunize patients without a Nova Scotia health card, and should use the dummy patient ID 7777777777. • All reasonable attempts should be made to obtain the Nova Scotia health card number, and this dummy ID should only be used in those cases where a patient does not have a valid Nova Scotia health card (e.g., students from out of province). • Claims should be submitted using the DIN of the vaccine administered to the patient, unless the patient is pregnant, or is a child receiving a second vaccine dose. In these instances the following PINS are to be used: o Pregnant Women – Agriflu® PIN 93899922 – Fluviral® PIN 93899921 o Second Dose for Children – Agriflu® PIN 93899920 – Fluviral® PIN 93899919 • The quantity entered for the claim should be per ml, as per the Standardization of Package Sizes section in the Pharmacists’ Guide. PAGE 3 OF 6 PHARMACISTS’ EDITION VOLUME 13-06 New Exception Status Benefits The following product has been reviewed by the Canadian Drug Expert Committee (CDEC) and will be listed as exception status benefit, with the following criteria effective December 1, 2013. PRODUCT STRENGTH DIN PRESCRIBER Eliquis® (apixaban) 2.5mg Tab 5mg Tab 02377233 02397714 DNP DNP BENEFIT STATUS E E MFR BRI BRI Criteria • Inclusion Criteria: At-risk patients with non-valvular atrial fibrillation (AF) who require apixaban for the prevention of stroke and systemic embolism AND in whom: - Anticoagulation is inadequate following at least a 2-month trial on warfarin; OR - Anticoagulation with warfarin is contraindicated or not possible due to inability to regularly monitor via International Normalized Ratio (INR) testing (i.e. no access to INR testing services at a laboratory, clinic, pharmacy, and at home) • Exclusion Criteria: Patients with impaired renal function (creatinine clearance or estimated glomerular filtration rate < 25 mL/min) OR ≥ 75 years of age and without documented stable renal function OR hemodynamically significant rheumatic valvular heart disease, especially mitral stenosis; OR prosthetic heart valves • Notes: a. At risk patients with non valvular atrial fibrillation are defined as those with a CHADS2 score of ≥ 1. Prescribers may consider an antiplatelet regimen or oral anticoagulation for patients with CHADS2 score of ≥ 1. b. Inadequate anticoagulation is defined as INR testing results that are outside the desired INR range for at least 35% of the tests during the monitoring period (i.e. adequate anticoagulation is defined as INR test results that are within the desired INR range for at least 65% of the tests during the monitoring period). c. Documented stable renal function is defined as creatinine or estimated glomerular filtration rate maintained for at least 3 months. d. Dosing: the usual recommended dose is 5mg twice daily; a reduced dose of apixaban 2.5mg twice daily is recommended for patients with at least two [2] of the following: age ≥ 80 years, body weight ≤ 60kg, or serum creatinine ≥ 133 micromole/litre. e. Since renal impairment can increase bleeding risk, renal function should be regularly monitored. Other factors that increase bleeding risk should also be assessed and monitored (see apixaban Product Monograph). f. Patients starting apixaban should have ready access to appropriate medical services to manage a major bleeding event. g. There is currently no data to support that apixaban provides adequate anticoagulation in patients with rheumatic valvular disease or those with prosthetic heart valves. As a result, apixaban is not recommended for these patient populations. PAGE 4 OF 6 PHARMACISTS’ EDITION VOLUME 13-06 New Exception Status Benefits – Continued… The following product has been reviewed by the pCODR Expert Review Committee and will be listed as exception status benefits, with the following criteria effective December 1, 2013. BENEFIT MFR STATUS Xalkori® (crizotinib) 200mg Cap 02384256 DNP E PFI 250mg Cap 02384264 DNP E PFI Criteria • As a second-line therapy for patients with ALK-positive advanced non-small cell lung cancer with ECOG performance status ≤ 2. PRODUCT STRENGTH Decision Highlights • DIN PRESCRIBER In previously treated patients, crizotinib was associated with an increase in progression free survival (7.7 months versus 3.0 months) and an improved quality of life versus standard of care chemotherapy. Criteria Update – rivaroxaban (Xarelto®) Please note that effective December 1, 2013, the criteria for Xarelto® will be updated to include the following: PRODUCT STRENGTH Xarelto® (rivaroxaban) DIN PRESCRIBER BENEFIT STATUS E E MFR 15mg Tab 02378604 DNP BAY 20mg Tab 02378612 DNP BAY New Criteria • Inclusion Criteria: Treatment of deep vein thrombosis (DVT) without symptomatic pulmonary embolism (PE) Coverage Period: up to 6 months • Notes: a. The recommended dose of rivaroxaban for patients initiating DVT treatment is 15mg twice daily for 3 weeks, followed by 20mg once daily. b. Drug plan coverage for rivaroxaban is an alternative to heparin/warfarin. When used for greater than 6 months, rivaroxaban is more costly than heparin/warfarin. As such, patients with an intended duration of therapy greater than 6 months should be considered for initiation on heparain/warfarin. • Since renal impairment can increase bleeding risk, it is important to monitor renal function regularly. Other factors that increase bleeding risks should also be assessed and monitored (see rivaroxaban product monograph) Decision Highlights • In one large randomized controlled trial of patients with DVT without symptomatic PE, rivaroxaban was non-inferior to a standard regimen of enoxaparin plus vitamin K antagonist. The majority of patients received treatment for six months or less; limited comparative clinical data is available for treatment durations exceeding six months. Treatment durations greater than six months are more costly than enoxaparin plus a vitamin K antagonist. PAGE 5 OF 6 PHARMACISTS’ EDITION VOLUME 13-06 Criteria Update – sunitinib (Sutent®) Please note that effective December 1, 2013, the criteria for Sutent® will be updated to include the following: PRODUCT BENEFIT MFR STATUS 12.5mg Tab 02280795 DNP E PFI 25mg Tab 02280809 DNP E PFI 50mg Tab 02280817 DNP E PFI New Criteria • For the treatment of patients with progressive, unresectable, well or moderately differentiated, locally advanced or metastatic pancreatic neuroendocrine tumors (pNET) with good performance status (ECOG 0-2), until disease progression STRENGTH Sutent® (sunitinib) Decision Highlights • DIN PRESCRIBER The Committee was satisfied there was a net clinical benefit based on the magnitude of the observed hazard ratio for risk of death and the observed progression-free survival difference between sunitinib and placebo (11.4 months versus 5.5 months) New Diabetic Products The following products are new listings to the Nova Scotia Formulary, effective December 1, 2013. The benefit status and reimbursement price within the Nova Scotia Pharmacare Programs is indicated. DNP BENEFIT STATUS SFD MSR 0.0490 DNP SFD MSR 0.0490 DNP SFD MSR PRODUCT DIN/PIN MRP PRESCRIBER Medi+Sure BG Test Strip 97799403 0.4900 Medi+Sure Soft 30G Twist Lancet (Purple) Medi+Sure Soft 33G Twist Lancet (Beige) 97799388 97799388 MFR Non-Insured Products The following products were reviewed by the Canadian Drug Expert Committee (CDEC) and were not recommended to be listed as insured benefits under the Nova Scotia Pharmacare Programs. PRODUCT STRENGTH DIN PRESCRIBER BENEFIT STATUS Not Insured MFR Apprilon® 40mg Cap 02373955 N/A GAL (doxycycline monohydrate) Decision Highlights • Addition clinical benefit of Apprilon® is uncertain and alternative treatments for inflammatory rosacea are currently available and are more cost-effective. PAGE 6 OF 6 PHARMACISTS’ EDITION VOLUME 13-06 Non Insured Products – Continued… The following product will not be insured in the Pharmacare Programs, however, it will be funded through the Exception Drug Fund (CDHA) as per other HIV medications. PRODUCT STRENGTH DIN PRESCRIBER Stribild ® (elvitegravir/cobicistat/ emtricitabine/tenofovir) 150mg/150mg/ 200mg/ 300mg Tab 02397137 N/A BENEFIT STATUS Not Insured MFR GIL The following product was reviewed by the pCODR Expert Review Committee for the treatment of soft tissue sarcoma (new indication) and the recommendation was not to list. PRODUCT STRENGTH DIN PRESCRIBER BENEFIT STATUS Not Insured MFR Votrient® 200mg Tab 02352303 N/A GSK (pazopanib) Decision Highlights • For the treatment of soft tissue sarcoma, compared with placebo, pazopanib conferred modest progression free survival, no overall survival benefit, no measured improvement in quality of life, and the therapy was not shown to be cost effective. Auditors Corner Audit Criteria for Administration of the Influenza Vaccine Required documentation to be available at the time of audit includes: • • Signed Patient Consent and Disclosure form Signed Confirmation of Agreement on file with Nova Scotia Pharmacare Pharmacy Closing or Transferring Ownership As indicated in the Tariff Agreement between the Pharmacy Association of Nova Scotia and the Nova Scotia Department of Health and Wellness, if your pharmacy is closing or changing ownership, it is your responsibility to notify our office within 30 days in advance of transfer/closing. This information will be retained in confidence. A close-out prescription audit is required. You may contact our office at msiproviders@medavie.bluecross.ca or 496-7011 or toll free 1-866-553-0585. DECEMBER 2013 • VOLUME 13-07 PHARMACISTS’ EDITION Nova Scotia Formulary Updates Nova Scotia Formulary Updates Updates to Minimum Day Supply Rule Updates to Minimum Day Supply Rule New Exception Status Benefits - Monurol Seebri New Benefits - Pataday Patanol Zaditor The following is a list of ATC categories that have been added to the existing ATC categories list for which refill claims for drugs and products must be for a minimum of 28 day supply. The complete list of all ATC categories for which refill claims must be for a minimum of 28 day supply will be in the next update of the Pharmacists' Guide. ATC CODE DESCRIPTOR A02A A02B Antacids Drugs for Peptic Ulcer and Gastroesophageal Reflux Disease (GERD) A06 Laxatives A07E Intestinal Anti-Inflammatory Agents A09 Digestives, Including Enzymes A11 Vitamins Standard Package Size Reminder B01AC Platelet Aggregation Inhibitors Excl. Heparin Standardization of Package Sizes B03 Antianaemic Preparations Publicly Funded Vaccines in Nova Scotia M01A Anti-Inflammatory/Antirheumatic Prod., Non-Steroids M04A Antigout Preparations N02BA01 Acetylaslicylic Acid N02BA11 Diflunisal N02BG04 Floctafenine N03AD Succinimide Derivatives N03AF Carboxamide Derivatives N03AG Fatty Acid Derivatives N03AX09 Lamotrigine N03AX11 Topiramate New Products Non-Insured Products - Aloxi Aloxi IV Samsca Sublinox Basic Medication Review Service Advanced Medication Review Service Changes to the Pharmacists' Guide PAGE 2 OF 8 PHARMACISTS’ EDITION VOLUME 13-07 Updates to Minimum Day Supply Rule Continued… ATC CODE DESCRIPTOR N03AX14 Levetiracetam N03AX18 Lacosamide N04 Anti-Parkinson Drugs N07C Antivertigo Preparations S01X Other Ophthalmologicals New Exception Status Benefits The following product has been reviewed by the Atlantic Expert Advisory Committee (AEAC) and will be listed as an exception status benefit with the following criteria, effective December 30, 2013. PRODUCT STRENGTH DIN PRESCRIBER BENEFIT STATUS E MFR Monurol® (fosfomycin 3 g/sachet 02240335 DNP TRI tromethamine) Criteria • For the treatment of uncomplicated urinary tract infections in adult female patients where: • The infecting organism is resistant to other oral agents [Criteria Code 01] OR • Other less costly treatments are not tolerated [Criteria Code 02] Decision Highlights • Monurol® (fosfomycin) is indicated for the treatment of uncomplicated urinary tract infections. It is not indicated for treatment of pyelonephritis or perinephric abscess. Although more expensive, fosfomycin is a useful option for patients intolerant and/or resistant to nitrofurantoin and TMP-SMX. • Note: The committee also noted that fluoroquinolones are not recommended for the treatment of uncomplicated urinary tract infections and should be reserved for severe infections or intolerance to other antibiotics. PAGE 3 OF 8 PHARMACISTS’ EDITION VOLUME 13-07 New Exception Status Benefits Continued… The following product has been reviewed by the Canadian Drug Expert Committee (CDEC) and will be listed as an exception status benefit with the following criteria, effective December 30, 2013. PRODUCT STRENGTH DIN PRESCRIBER BENEFIT STATUS E MFR Seebri® (glycopyrronium 50mcg Cap for 02394936 DNP NVR bromide) Inh Criteria • For the treatment of chronic obstructive pulmonary disease (COPD), if symptoms persist after 2-3 months of short-acting bronchodilator therapy (i.e., salbutamol at a maximum dose of 8 puffs/day or ipratropium at maximum dose of 12 puffs/day) • Coverage can be provided without a trial of short-acting agent if: • There is spirometric evidence of at least moderate to severe airflow obstruction, (i.e., postbronchodilator values FEV1 < 60% and FEV1/FVC ratio < 0.7), and significant symptoms (i.e., MRC score of 3-5**) • combination therapy with glycopyrronium and a long-acting beta2 agonist/inhaled corticosteroid will only be considered if: • there is spirometric evidence of at least moderate to severe airflow obstruction (postbronchodilator values FEV1 < 60% and FEV1/FVC ratio < 0.7), and significant symptoms (i.e., MRC score of 3-5**) AND • there is evidence of one or more moderate-to-severe exacerbations per year, on average, for 2 consecutive years requiring antibiotics and/or systemic (oral or intravenous) corticosteroids ** Canadian Thoracic Society COPD Classification By Symptom/Disability: Moderate - (MRC 3-4) Shortness of breath from COPD causing the patient to stop after walking about 100 meters (or after a few minutes) on the level. Severe - (MRC 5) Shortness of breath from COPD resulting in the patient being too breathless to leave the house or breathless after undressing, or the presence of chronic respiratory failure or clinical signs of right heart failure. MRC= Medical Research Council Dyspnea Scale Decision Highlights • Seebri Breezehaler® (glycopyrronium) is an alternative long-acting anticholinergic for the treatment of COPD in patients who meet the same eligibility criteria as tiotropium. GLOW-1, GLOW-2, and a network meta-analysis suggested that glycopyrronium and tiotropium have similar efficacy for improving lung function in patients with COPD. PAGE 4 OF 8 PHARMACISTS’ EDITION VOLUME 13-07 New Benefits Based on a review by the Atlantic Expert Advisory Committee (AEAC), effective December 30, 2013, the following products will be listed as benefits under the Nova Scotia Pharmacare Programs. DNP BENEFIT STATUS SF ALC 02233143 DNP SF ALC 02242324 DNP SF NVO PRODUCT STRENGTH DIN PRESCRIBER Pataday 0.2% Oph Sol 02362171 Patanol 0.1% Oph Sol Zaditor 0.25% Oph Sol MFR New Products Based on a review by the Atlantic Expert Advisory Committee (AEAC), effective December 30, 2013, the following products will be listed as benefits under the Nova Scotia Pharmacare Programs. Where applicable, established coverage criteria will apply. DNP BENEFIT STATUS E FRS 02254603 DNP E FRS 02254638 DNP E FRS 02254646 DNP E FRS 02240344 DNP Not Insured PMS 02320681 DNP E JAN 02371081 DNP E MRZ PRODUCT STRENGTH DIN PRESCRIBER Pegetron Clearclick1 80mcg/0.5mL Inj/200mg Cap 100mcg/0.5mL Inj/200mg Cap 120mcg/0.5mL Inj/200mg Cap 150mcg/0.5mL Inj/200mg Cap 25mg Tab 02254581 90mg/1.0mL Syringe Inj 50 LD50 units/vial Pegetron Clearclick1 Pegetron Clearclick1 Pegetron Clearclick1 Sialor Stelara Xeomin MFR 1 Pegetron Clearclick is the replacement product for Pegetron Redipen. The Clearclick injector device is the only difference between the products. Non-Insured Products The following products were reviewed by the Canadian Drug Expert Committee (CDEC) and were not recommended to be listed as benefits under the Nova Scotia Pharmacare Programs. PRODUCT STRENGTH DIN PRESCRIBER Aloxi® (palonosetron) 0.5mg Cap 02381729 N/A BENEFIT STATUS Not Insured MFR EIS Decision Highlights • Clinical evidence supporting oral palonosetron superiority to oral ondansetron was not established and the costs of oral palonosetron are higher. PAGE 5 OF 8 PHARMACISTS’ EDITION VOLUME 13-07 Non-Insured Products Continued… PRODUCT STRENGTH DIN PRESCRIBER Aloxi IV® (palonosetron) 0.25mg/5mL Inj 02381710 N/A BENEFIT STATUS Not Insured MFR EIS Decision Highlights • The cost-effectiveness is uncertain. BENEFIT MFR STATUS Samsca® (tolvaptan) 15mg Tab 02370468 N/A Not Insured OTS 30mg Tab 02370476 N/A Not Insured OTS Decision Highlights • Tolvaptan was not considered to be cost-effective in patients with heart failure and non-hypovolemic hyponatremia, and there was insufficient evidence that treatment with tolvaptan provides clinical benefits for mortality, morbidity, or reduced length of hospitalization relative to alternatives or placebo. PRODUCT STRENGTH DIN PRESCRIBER PRODUCT STRENGTH DIN PRESCRIBER Sublinox® (zolpidem tartrate) 5mg SL Tab 10mg SL Tab 02391678 02370433 N/A N/A BENEFIT STATUS Not Insured Not Insured MFR MVL MVL Decision Highlights • There are no studies comparing sublingual zolpidem against other treatments for insomnia that are currently marketed in Canada; therefore, there is insufficient evidence to determine clinical benefit versus other hypnotics for the treatment of acute, short-term insomnia. Basic Medication Review Service Basic Medication Review Service (BMRS) – approximately 20 to 30 minutes to complete - is an insured service under all the Pharmacare Programs, except the Under 65 – LTC Program. To qualify for the program: • The individual must be a beneficiary of a Nova Scotia Pharmacare Program, except the Under 65 – LTC Program. • The beneficiary must agree with their pharmacist that they are a suitable candidate for the program and sign a consent form which, along with all other documentation, is to be kept on file in the pharmacy for at least three years for audit purposes. • The beneficiary must not reside in a nursing home, home for special care, or be receiving medication in compliance packaging. • The beneficiary must meet with the pharmacist for an in-person consultation. • The beneficiary must be taking 3 or more prescription medications that are used for the treatment of chronic conditions, and are covered by the Pharmacare Programs. • The beneficiary must be provided with a comprehensive drug review list that is dated and authorized with the pharmacist’s and the patient’s signatures. PAGE 6 OF 8 PHARMACISTS’ EDITION VOLUME 13-07 Advanced Medication Review Service Advanced Medication Review Service (AMRS) – approximately one and one-half hours to complete - is an insured service under the Nova Scotia Seniors’ Pharmacare Program. Pharmacies are required to complete the Pharmacy sign-up form and fax it to the Pharmacy Association of Nova Scotia (PANS) prior to offering the service to their patients.* It is important for the pharmacy to be registered for billing and audit purposes. To qualify for the program, beneficiaries must: • • • • • Be beneficiaries of the Nova Scotia Seniors’ Pharmacare Program. Agree with their pharmacist that they are a suitable candidate for the program. A signed consent form with the pharmacist’s and patient’s signatures, and all documentation are to be kept on file in the pharmacy for at least three years for audit purposes. Not reside in a nursing home, home for special care, or be receiving medication in compliance packaging. Be taking 4 or more prescription medications; OR taking one of the following: - methyldopa - indomethacin - cyclobenzaprine - diazepam - chlordiazepoxide - clorazepate - amitriptyline Have at least one of the following diseases: - asthma - diabetes - hypertension - hyperlipidemia - congestive heart failure - chronic obstructive pulmonary disease - arthritis Standard Package Size Reminder The Nova Scotia Pharmacare Programs has been receiving a significant number of claims with incorrect entries in the “Quantity” field. Some of the more common examples are listed below: QUANTITY ENTRIES PRODUCT Remicade (infliximab) DOSAGE FORM Powder for injection INCORRECT 100 mg CORRECT 1 vial Lantus (insulin glargine) 100 U/mL cartridge 1500 U, 5 Vials, 1 Box 15 mL Lantus (insulin glargine) Solostar 3mL Pens 1500 U, 5 Pens, 1Box 15 mL PAGE 7 OF 8 PHARMACISTS’ EDITION VOLUME 13-07 Standardization of Package Sizes Providers are reminded that claims to the Pharmacare Programs must be billed according to the following Standardized Package Sizes. FORM QUANTITY FORM QUANTITY Aerosols Per dose Nasal sprays Per dose Capsules Per capsule Nebules Per ml Creams Per gram Ointments Per gram Enemas Per ml Oral contraceptives As 21 or 28 Gels Per gram Ostomy supplies Per item (e.g., 20 pouches) Inhalers Per dose Patches Per patch Insulins (vials, penfills, cartridges) Per ml Powders Per gram Kits Per kit Powder Injectables Per vial Lancets Per lancet Suppositories Per suppository Liquids Injectables Per ml Tablets Per tablet Liquids (except methadone) Per ml Testing strips Per testing strip Liquid methadone Per mg Other: FORM QUANTITY Package/Kits of more than one drug Per package (e.g., HP-Pac®, Monistat 3 Dual-Pack®, Didrocal®) Per test strip (e.g., Sidekick® Blood Glucose Testing System) Packages of blood glucose testing strips with built-in meter Publicly Funded Vaccines in Nova Scotia The new Pharmacy Act and associated regulations including the Registration, Licensing and Professional Accountability Regulations and the Pharmacy Practice Regulations were approved by the provincial government and came into effect August 6, 2013. With the development and approval of the standards of practice by the Nova Scotia College of Pharmacists (NSCP), pharmacists now have the authority to administer drugs by injection provided they meet the required training and application expectations set out by the Regulations and Standards of Practice. With this change, a dedicated supply of publicly funded influenza vaccine was made available to participating pharmacies as part of the provincial immunization program. The influenza vaccine is available free of charge to all Nova Scotians from many health care providers, including pharmacists. PAGE 8 OF 8 PHARMACISTS’ EDITION VOLUME 13-07 Publicly Funded Vaccines in Nova Scotia Continued… Although the influenza vaccine is the only publicly funded vaccine that can be accessed through community pharmacies, there are other vaccines that pharmacies can provide to their patients. In order to ensure that all patients are aware of which vaccines are publicly funded and available free of charge from other immunization providers, Public Health has included information in the NS Immunization Manual. Please refer to chapter ten for information on public funded vaccines using the following link: http://novascotia.ca/dhw/cdpc/documents/Immunization-Manual.pdf With these documents you can help ensure patients have the most up to date information on which vaccines are eligible free of charge from other immunization providers in Nova Scotia, and if applicable, any specific conditions that may apply. Any questions on these documents should be directed to your local Public Health office. Changes to the Pharmacists' Guide Please note that the Pharmacists' Guide will no longer be provided in print form. An electronic version of the Pharmacists' Guide is available on the Pharmacare website. The next update will be available online in January, 2014.