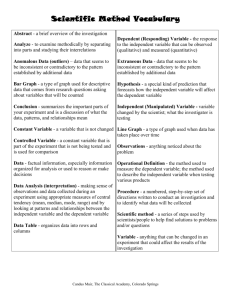
New Haven CAPT Practice Test
advertisement

CAPT PRACTICE (RELEASED ITEMS) Power Plants A power company is building a new power plant to provide electricity for several communities. A group of students was studying simple electromagnets. They carried out the following experiment. 1. Take a nail and wrap a 10-­‐cm wire around the nail five times. 2. Connect both ends of the wire to a 1.5-­‐volt battery. 3. Measure how many paper clips can be lifted by the end of the nail. 4. Repeat for three trials. 5. Repeat steps 1–4 using the same wire and increasing the number of loops of wire around the nail by five. Number of Paper Clips Lifted Number of Loops of Wire Trial 1 Trial 2 Trial 3 Average 5 4 2 3 3.0 10 4 5 5 4.7 15 7 5 6 6.0 (I: DINQ4) Which of the following would most improve the design of the experiment? a. Replace the nail with a wood pencil. b. Increase the length of the wire as the number of loops is increased. c. Keep the number of paper clips lifted constant in the experiment. d. Increase the number of loops of wire beyond fifteen. (I: DINQ8) Which graph correctly displays the results of the experiment? (I: D9 )What is a major advantage of using wind energy instead of coal or nuclear power plants? f. Wind is a renewable energy source. g. Wind is consistently available in all locations. h. Windmills reduce the strength of severe storms. j. A single windmill produces more energy than a nuclear plant. -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Static (I D4) To demonstrate static electricity, a teacher takes an inflated rubber balloon and rubs it on his head. The rubber balloon picks up electrons from his hair, which causes his hair to have a(n) ____________. f. electrical current g. net positive charge h. net negative charge j. buildup of magnetic energy -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ In the Kitchen Common kitchen appliances include electric stoves, toasters, and blenders. Each appliance uses an energy source and involves energy changes to prepare food. ( I: D1). An open pot of water is heated on the stove. As water boils, the molecules _________. f. move slower and closer together g. move faster and farther apart h. get larger j. get smaller (I: D5). When in use, the heating element in a toaster glows and gives off heat. This is because atoms within the heating element _____________. a. undergo chemical reactions b. are excited by the flow of electrons c. gain electrons and increase in temperature d. conduct light and heat from the outlet A group of students carried out the following investigation. “Our hypothesis is that the greater the wire diameter used in a toaster, the greater the resistance in the wire.” 1. We took a 4-­‐meter length of wire with a diameter of 0.5 millimeters. 2. We attached the wire to a 3-­‐volt battery and measured the current. 3. Knowing the voltage and current, we calculated the resistance in the wire. 4. We repeated the same steps with wires of increased diameter. 5. We organized our data in the table below. Diameter of Wire Measured Calculated (millimeters) Current (milliamps) Resistance (ohms) 0.5 1.0 1.5 2.0 2.5 10 40 80 100 250 300.0 75.0 37.5 30.0 12.0 (I: DINQ7) To be certain that data in the table are correct, you will have to _____________. f. go online and seek additional information g. ask for your teacher’s opinion h. repeat the experiment as described j. repeat the experiment with different variables -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Recreation Center Pool A local recreation center has received funding to build a swimming pool. After construction, the center will be responsible for all costs associated with pool operation. As a result, the center must consider a variety of design options, including pool size, location and heating. (I D1) What happens to water molecules in a pool as they absorb energy? f. The molecules occupy less volume. g. The molecules begin to move more slowly. h. The kinetic energy of the atoms decreases. i. The rate of collision between molecules increases. (I DINQ5) Prior to pool construction, engineers use computer models to compare which of several pool designs require the least amount of energy to be heated. What is the dependent variable in the computer models? a. pool size b. pool shape c. pool location d. pool temperature (I D2) Where should hot water enter the pool to better heat the water? f. A g. B h. C j. D Electricity (I D INQ.6 ) A student coils a bare copper wire around a metal rod and attaches the ends of the wire to an ammeter. He quickly moves a magnet past the coil and notes the resulting current. How could the student alter this apparatus to create a larger current? a. use thinner wire b. use insulated wire c. increase the length of the rod that is used d. increase the number of times the wire is coiled around the rod (I D 7 ) The picture below shows a turbine generator used to produce electricity at a geothermal power plant. Electricity is produced by using steam to _____________. f. heat the turbine generators g. spin the turbine generators h. reduce friction in the turbine generators j. reduce emissions from the turbine generators -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Plastics Consumers use many products made of plastic. Plastics are carbon-­‐based polymers made from smaller carbon compounds, called monomers. (II: D13) In organic molecules, the carbon atoms and the hydrogen atoms are held together by _____________. f. hydrogen bonds g. covalent bonds h. ionic bonds j. nuclear bonds A company is considering polymers A and B below for the production of plastic shopping bags. (II: D15) Which polymer is more appropriate for the production of shopping bags? a. Polymer A, because its branched structure provides greater strength b. Polymer A, because its branched structure provides greater flexibility c. Polymer B, because its linear structure provides greater strength d. Polymer B, because its linear structure provides greater flexibility (II: D18) Many communities encourage the recycling of plastics, even though it is often expensive to do so. Why is it beneficial to the environment to recycle plastics? f. Plastics are expensive to manufacture. g. Plastics are made from renewable resources. h. Plastics decompose quickly, releasing toxic chemicals. j. Plastics decompose slowly, taking up space in landfills. Rubber Tires The tires on most cars are not made of natural rubber because it becomes brittle in the cold and sticky in the heat. Instead, natural rubber is vulcanized by adding sulfur and heat, making it stronger and more elastic. This process is represented chemically in the diagram below. (II: D16) During the vulcanization reaction shown above, the natural rubber polymer is converted to a new polymer by the _____________. a. cross-­‐linking of carbon atoms with sulfur atoms b. cross-­‐linking of hydrogen atoms with sulfur atoms c. replacement of carbon atoms with sulfur atoms d. replacement of hydrogen atoms with sulfur atoms (II: D14) The complete combustion or burning of natural rubber will produce _______. f. hydrogen and oxygen g. oxygen and water h. hydrogen gas and water j. carbon dioxide and water -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Polymers (II D 16.) The pictures below show the structures of two polymers. What can be concluded from comparing these two pictures? a. Polymers are easily broken down into their component parts. b. The same number of carbon atoms may be arranged in various ways. c. Linear polymer structures are stronger than ringed polymer structures. d. It takes fewer monomers to form a linear polymer than a ringed polymer. (II D INQ1) Researchers have developed a biopolymer made from orange peels and carbon dioxide. According to the researchers, using CO2 to make polymers could reduce the amount of greenhouse gas emitted into the atmosphere. What question would an environmentalist most likely want answered before accepting this statement as credible? f. How long will it take the biopolymer to decompose? g. Is the biopolymer as strong as hydrocarbon polymers? h. Can other types of citrus be used to produce biopolymers? j. What happens to the CO2 when the biopolymer decomposes? -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Petroleum-­‐based Polymers vs. Plant-­‐based Polymers A petroleum-­‐based (inorganic) polymer is commonly used for grocery bags. Recently there has been a push by environmentalists to make grocery bags out of plant-­‐based (organic) polymers. Students in a science class decided to investigate the strength of the two types of polymers. They obtained one petroleum-­‐based (inorganic) polymer bag and one plant-­‐based (organic) polymer bag of the same size and thickness. They added 100-­‐gram weights to each bag until it broke. (II DINQ5) What is the independent variable in the investigation? a. the size of the bags b. the type of polymer c. the thickness of the bags d. the amount of weight of the bags (II DINQ4) The students found that the plant-­‐based polymer grocery bag held 500 grams before breaking and the petroleum-­‐based polymer grocery bag held 600 grams before breaking. In order to increase confidence in their results, the students should repeat the investigation using _____________. f. only plant-­‐based polymer bags g. two other types of polymer bags h. a double thickness of each polymer bag j. both the plant and petroleum polymer bags -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Experimental Data The following data are recorded during a supervised investigation. (II: DINQ1) What question was the investigator most likely trying to answer? a. How does the presence of oxygen affect combustion? b. At what point is equilibrium reached in a combustion reaction? c. What are the byproducts of an incomplete combustion reaction? d. Does the amount of fuel in a combustion reaction affect the burn time -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ The London Smog Disaster of 1952 On December 5, 1952, London, England, experienced temperatures that were much colder than normal. As a result, large amounts of coal were burned in furnaces to keep residences warm. This occurred at the same time as the formation of a heavy fog. Water from the fog condensed around airborne soot particles, and a thick smog quickly developed. Nearly 12,000 human deaths resulted. (III D 22) In addition to soot, what product of the burning coal contributed most to the extreme pollution of London’s air? a. uranium (U) b. methane (CH4) c. sulfur dioxide (SO2) d. chlorofluorocarbons (CFCs) (III D 26) Which government action was most likely the result of the London smog disaster of 1952? f. establishment of youth curfews after dark g. creation of a privately funded healthcare system h. conversion from underground mining for coal to strip mining for coal j. provision of grants for homeowners to convert to gas or oil-­‐fueled heaters (III D INQ.7) The graph below shows the correlation between pollutants and human deaths during the London smog disaster of 1952. Which conclusion is best supported by the data? a. Acid rain fell from December 4 to December 10. b. Smoke caused more deaths than sulfur dioxide. c. Sulfur dioxide remains in the air longer than smoke. d. Air pollution peaked between December 7 and December 8. -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Pollution Pollution has many causes and can affect air and water quality in a variety of ways. (III: D23) The burning of fossil fuels may contribute to an increase in global temperatures. What might lead to this increase in temperature? a. The combustion products reflect solar radiation away from Earth. b. Carbon dioxide in the atmosphere attracts solar radiation. c. Carbon dioxide in the atmosphere blocks energy from escaping into space. d. The combustion products allow more energy to enter the earth. (III: D22) Which of the following is directly responsible for acid rain? f. steam vented from a nuclear power plant g. sulfur dioxide released from a coal-­‐fired power plant h. mining of coal for a coal-­‐fired power plant j. processing of uranium for a nuclear power plant (III: DINQ4) A student wanted to design an experiment to determine the effect of nitrates on algae growth. Which procedure would create the most valid results? a. Vary both the temperature and the amount of nitrates. b. Keep the temperature constant and vary the amount of nitrates. c. Vary the temperature and keep the amount of nitrates constant. d. Keep both the temperature and the amount of nitrates constant. -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Water Cycle (III D20) Over 6 billion people on Earth use water every day, yet Earth’s water supply remains relatively constant. This is because _____________. a. the sea level is rising b. water exists in three phases on Earth c. water is constantly recycled by the hydrologic cycle d. global warming melts ice to replace water that is used -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Global Warming The burning of fossil fuels to heat homes, power factories, and run automobiles is largely responsible for increasing carbon dioxide emissions. Many scientists hypothesize that the increase in these greenhouse gases contributes directly to global warming. The graph below shows average global changes in carbon dioxide and temperature over an extended period of time. (III D 23) The natural greenhouse is a phenomenon that is beneficial as it results in _____________. a. the maintenance of Earth’s temperatureµ b. a thinning of Earth’s atmospheric ozone layer c. an increase in the amount of carbon dioxide in Earth’s atmosphere d. the bending of the rays of sunlight that penetrate Earth’s atmosphere (III DINQ2) A business claims to be doing “everything possible” to reduce greenhouse gas emissions. Which company practice would cause a consumer to question this claim? a. The company vehicles all use diesel fuel.µ b. The building lights are triggered by motion. c. The building is powered by geothermal energy. d. The company purchases recycled paper products. (III DINQ8) Assume the use of fossil fuels continues to increase over the next decade. What prediction are scientists most likely to make for carbon dioxide and temperature change? a. Carbon dioxide will increase, causing an increase in temperature.µ b. Temperature will increase, causing a decrease in carbon dioxide. c. Carbon dioxide will increase, and temperature will remain the same. d. Temperature will increase, and carbon dioxide will remain the same. -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Global (III DINQ3) The picture below shows the extent of summer Arctic Sea ice in 1979 and 2005. Which hypothesis is best supported by the changes in sea-­‐ice coverage? a. Earth’s climate is gradually warming. b. Arctic Sea ice is migrating away from Earth’s poles. c. Global warming is caused by human activity, not nature. d. Global warming occurs only at Earth’s poles during the summer. (III D23) Which of the following pictures best represents the natural greenhouse effect? -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Carbon Cycle The diagram below shows carbon cycling associated with oil and gas consumption. (III: D19) Which arrow on the carbon cycle diagram represents the process that takes the longest amount of time to occur? f. 1 g. 3 h. 5 j. 7 (III:DINQ2) A teacher provides her class with a table displaying the relative greenhouse effect per molecule of different gases compared to carbon dioxide. Carbon Nitrous Methane CFCs Dioxide Oxide 1 30 times 160 times 17,000 times Based on this table, a student made the conclusion that carbon dioxide is not the main cause of the greenhouse effect. What other data are needed to make a stronger conclusion? a. data about the origin of the gases b. data about the size of each type of molecule c. data about the absorption of these gases by plants d. data about the amount of each gas in the atmosphere -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Cells (IV D 30.) Depending on its electric charge, shape, and chemical properties, a substance may or may not be allowed to pass through a cell membrane. This function of the cell membrane is important because it _____________. a. prevents cell division b. prevents destruction of the cell wall c. allows the cell to maintain homeostasis d. allows amino acids to move into and out of the cell (IV D INQ.9) Students placed a sample of red blood cells (RBC) and a sample of skin cells in 2 test tubes that contained the same glucose solution. After 24 hours, the students observed the cells under the microscope and found that the cells in both samples increased in size. What conclusion might be drawn from this observation? f. The cytoplasm of the red blood cells is more concentrated than that of the skin cells. g. Skin cells absorb water faster than the red blood cells. h. Both cells absorb water when placed in the glucose solution. j. Both cells absorb water when placed in any solution. -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Plant Cells Plants, like all other organisms, are composed of cells. (IV: D30) A group of students placed spinach leaves in a beaker of water in full sunlight. After several hours, small bubbles appeared on the leaves. These bubbles probably consisted of _____________. a. H2O b. O2 c. CO2 d. H2 (IV: D27) Generally, plants that grow in the shade have larger leaves in comparison to plants that grow in full sun. The advantage of having larger leaves in a shaded environment is _____________. f. an increase in water supply g. an increase in light absorption h. a decrease in water loss j. a decrease in heat production Students are exploring what happens to potatoes when placed in liquid. They cut one potato into slices and placed the slices in 3 different solutions, as described in the table below. Solution 100 cc distilled water 100 cc saltwater A 100 cc saltwater B Amount of Initial Mass of Mass of Potato after Solute (in grams) Potato (in grams) 25 Minutes (in grams) 0 10 12 7 10 10 25 10 8 (IV: DINQ 5) Which of the following is the independent variable in the students’ experiment? a. the amount of time in the solution b. the shape of the slices c. the mass of the potatoes d. the concentration of the solutions -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Genetics (IV D23) Two farmers plant different varieties of corn on neighboring farms. Farmer A plants genetically modified corn. Farmer B plants a non-­‐modified variety of corn. What would be farmer B’s primary concern if she plans to gather seed for next year’s crop? f. loss of genetic variability in the non-­‐modified variety g. that mutation rates will increase in the non-­‐modified variety h. that insects will only pollinate the genetically modified corn j. unintended transfer of modified genes to her crop by cross-­‐pollination (IV D32)What is accomplished by treating a person who has a bacterial infection with antibiotics? a. immunity to future infections b. weakening of the person’s immune system c. reduction in the duration and intensity of the infection d. modification of bacterial DNA to make the bacteria harmless (IV DINQ7) The figures below show the reaction rate of a specific enzyme at different temperatures and different pHs. What can be concluded about the enzyme? f. The enzyme works best at a pH of 8 and a temperature of 25°C. g. The enzyme only works at a pH of 8 and a temperature of 25°C. h. The enzyme is used up at a pH of 11 and a temperature of 35°C. j. The enzyme works better at a pH of 8 than a temperature of 25°C. -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Potato Blight Blight is a plant disease caused by a fungus that affects potato plants. Some wild breeds of potato have natural resistance to the fungus. These wild potatoes contain chemical compounds that cause them to taste bad. Scientists are trying to produce potato plants that are resistant to blight but still produce potatoes that taste good. (IV: D27) Which of the following describes an important difference between a potato plant cell and a human cell? a. Plant cells have a cell wall, and animal cells do not. b. Animal cells store water inside, and plant cells do not. c. Plant cells have a cell nucleus, and animal cells do not. d. Animal cells perform respiration, and plant cells do not. (IV: D35) The development of a blight-­‐resistant potato breed might be good for the environment because the new potato breed will need _____________. f. less water g. less fertilizer h. less fungicide j. less field space -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Typhoid Mary Mary Mallon was the first known “healthy carrier” of typhoid fever in the early 1900s. Health officials in New York investigated the households in which she worked as a maid and determined that she had transmitted the disease to dozens of people. Typhoid fever is caused by the bacterium Salmonella typhi. It is generally transmitted by eating food and drinking water that has come into contact with contaminated fecal matter. Symptoms of typhoid fever include headache, fever, diarrhea, and loss of appetite. (IV D23) Typhoid fever is best treated with _____________. a. surgery b. vaccines c. antibiotics d. gene therapy (IV DINQ.3) In 1910, Mary Mallon was banned from ever working in kitchens again. Five years later, health officials suspected that Mary had violated the ban. What most likely alerted health officials to the fact that Mary might be working in kitchens again? a. a new typhoid outbreak in New York b. the fact that she changed her name to Mary Brown c. identification of other healthy carriers in New York d. discovery of the typhoid bacterium on local vegetables -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Yeast Laboratory Investigation In a laboratory investigation, a student mixes 1 cup of warm water (30°C) with 30 grams of sugar and 5 grams of yeast. She pours the mixture into a glass bottle and secures a balloon over the opening. After several minutes, she observes that the balloon begins to inflate, as shown in the picture below. The student performs two additional trials. In trial 2 she uses water at 25°C, and in trial 3 she uses water at 20°C. She observes that the colder the water, the longer it takes the balloon to inflate. (IV DINQ3) After reviewing her data, the student decides to perform an additional trial at 35°C. She observes that the balloon inflates faster than during the trial in which the 30°C water was used. This additional trial supports which of the following hypotheses? f. Warmer temperatures are more favorable for yeast fermentation. g. Yeast require less sugar when maintained at lower temperatures. h. The optimum temperature for yeast fermentation is less than 35°C. j. The time required for fermentation increases with increasing temperature. (IV DINQ8) The graph below shows changes in a yeast population over the course of several days. The yeast were placed on a nutrient dish and allowed to grow. On which day was additional nutrient most likely added to the yeast culture? a. 3 b. 4 c. 6 d. 7 -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Illnesses The common cold is caused by a virus that enters the human body and causes mild, flu-­‐like symptoms. Some people believe that the common cold can be treated by digesting the herb Echinacea. The following table shows results from a study conducted to explore the effects of Echinacea on children with colds. Echinacea Study (V: DINQ2) Data in the table show that the use of Echinacea can _____________. f. reduce the length of cold infection from 10 to 7 days g. increase the incidence of colds in children from 52% to 64% h. increase the percent of children with skin rash from 2.7% to 7.1% j. reduce the numbers of children having colds from 370 to 337 cases (V: DINQ 9) A possible conclusion from the data is that Echinacea _____________. a. is a safe remedy for the common cold b. is effective only for children c. has side effects d. reduces the length of colds (V: D 40) It is very difficult to develop a vaccine against the common cold. The reason for this is that the common cold virus _____________. f. hides in the digestive system g. changes rapidly due to high mutation rates h. includes RNA as its genetic materials j. is too small for the immune system to detect -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Fossil Record (V: D44) The growth rate of a local population is dependent on the birth rate minus the death rate and ____________. f. the ratio of males to females in the population g. the lifespan of females beyond the reproductive age h. the amount of genetic variation that exists in the population j. the immigration and emigration of individuals to and from the population (V: D41). Vestigial structures, such as hip bones in whales and appendixes in humans, are those that have little or no function for the organism. What is the most likely reason for this loss of function over time? a. The organism is undergoing speciation. b. The organism is experiencing genetic drift. c. The structure was over utilized by the organism. d. The structure was not highly beneficial to the organism. The diagram below shows the fossil record of different species. (V: DINQ 7) When did a major extinction event most likely occur? f. at the end of the Cenozoic g. at the end of the Permian h. at the beginning of the Silurian j. at the beginning of the Cambrian (V: DINQ7) . According to the records of fossil species V and W, which statement is most likely true? a. Fossil species W appeared before fossil species V, allowing fossil species W to survive longer. b. Fossil species W was ancestral to fossil species V because it appeared before fossil species V. c. Fossil species W had greater genetic variability than fossil species V, allowing fossil species W to adapt and survive longer. d. Fossil species W had lower reproductive success than fossil species V, allowing smaller populations to adapt and survive. -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ Cystic Fibrosis Cystic fibrosis (CF) is a condition characterized by difficulty in breathing and digestion. CF is caused by a defect in a specific gene. The pedigree diagram below shows the inheritance pattern of cystic fibrosis in two generations of a family. (V D 37) Which couple has a 25% probability of producing offspring who are homozygous for cystic fibrosis? a. 3 and 4 b. 5 and 6 c. 7 and 8 d. 9 and 10 (V D 39) .An individual with CF is not able to transmit the disease by physical contact because _____________. a. the gene for the disorder is only carried in the bloodstream b. CF is a genetic disorder and can only be passed from parent to offspring c. the bacteria that transmit the defective gene must be inherited from a parent d. CF is so rare that the probability of coming into contact with an affected individual is low -­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐-­‐ (V D36) A scientist conducted a study of an organism and found that its body cells contained 40 chromosomes. These cells were cultured in the laboratory, and cell division was observed. What difference, if any, would the scientist expect to observe between body cell division and sex cell division in the organism? a. Body cells divide by mitosis, and sex cells divide by meiosis.µ b. Body cells divide by meiosis, and sex cells divide by mitosis. c. There is no difference; body cells and sex cells both divide by mitosis. d. There is no difference; body cells and sex cells both divide by meiosis. (V DINQ9) The graphs below show the annual number of AIDS deaths in the United States and in South Africa from 1999–2003. What conclusion is best supported by the data in the graphs? a. AIDS has been cured in the United States but not in South Africa. b. AIDS has caused a greater population decline in South Africa than it has in the United States.µ c. The number of AIDS deaths in each country is solely responsible for the population growth rate in each country. d. The population in South Africa has increased regardless of AIDS, whereas the United States population has decreased as a result of AIDS. (IV D 32) CAPT Science Open-­‐Ended Item: Enzymes1 Science students conducted an investigation to determine how enzymes affect apple juice production. Procedure: 1. Place coffee filter in paper cone, cut off 2 cm of the bottom of the cone, leaving a small hole. 2. Place 30 mL of applesauce into measuring cup, add 5 drops of enzyme A solution, and stir thoroughly. 3. Place a graduated cylinder under paper cone and add applesauce to coffee filter, stirring every minute. 4. Measure volume of apple juice in cup after 5 minutes using graduated cylinder. 5. Repeat steps 1– 4 for a second trial. 6. Repeat steps 1–5 using enzyme B solution. 7. Repeat steps 1–5 using water. Amount of Juice Produced a) What conclusion can be drawn from the students’ experiment and results? b) Assess the reliability of the results of this investigation. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: (V DINQ2) Genetically Modified Food Suppose a consumer reads the following news release regarding the safety of a genetically modified (GM) food product. GM Grains Pose No Health Risk Researchers report that genetically modified (GM) grains fed to test mice have no negative impact on health. In two trials, the offspring of mice fed GM grain for three weeks showed a similar survival rate as the offspring of mice that were fed non-­‐GM grain. The trials have been called as a victory for GM food producers. A spokesperson for the research group stated that “it is highly unlikely for any unintended side effects to occur as a result of human consumption of GM grains.” Provide three reasons a consumer should question the conclusions presented in this news release. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: Acid Rain (III DINQ4) A group of students wrote the following procedure for their investigation. Procedure: 1. Determine the mass of four different samples. 2. Pour vinegar in each of four separate, but identical, containers. 3. Place a sample of one material into one container and label. Repeat with remaining samples, placing a single sample into a single container. 4. After 24 hours, remove the samples from the containers and rinse each sample with distilled water. 5. Allow the samples to sit and dry for 30 minutes. 6. Determine the mass of each sample. The students’ data are recorded in the table below. Starting Ending Difference in Sample Mass (g) Mass (g) Mass (g) Marble 9.8 9.4 –0.4 Limestone 10.4 9.1 –1.3 Wood 11.2 11.2 0.0 Plastic 7.2 7.1 –0.1 After reading the group’s procedure, describe what additional information you would need in order to replicate the experiment. Make sure to include at least three pieces of information. Write your answer in your answer booklet CAPT Science Open-­‐Ended Item: Solar Cooker1 Investigation (I DINQ 6) Solar Cooker1 Investigation A group of students has designed a solar cooker for an investigation. They are investigating whether the material that a container is made of has an effect on the rate of temperature change over time. They obtain three containers of identical size. They add water to each container. The containers are placed inside the solar cooker, which is made of a box lined with aluminum foil. a) Identify two additional pieces of equipment that the students will need to use in their investigation. b) Explain why each piece of equipment is necessary. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: Polymer Investigation Polymer Investigation I (II DINQ5) A group of students wrote the following procedure for their investigation. Procedure: 1. Tightly wrap a sample of kitchen wrap from manufacturer A over the top of a coffee can. 2. Place a 10-­‐gram weight on the kitchen wrap to see if it breaks. 3. Continue to add 10-­‐gram weights one at a time until the kitchen wrap breaks and the weights fall into the can. 4. Record the number of 10-­‐gram weights the kitchen wrap held before breaking. 5. Repeat the procedure exactly for a sample of kitchen wrap from manufacturer B. 6. Repeat the procedure exactly for a sample of kitchen wrap from manufacturer C. a) What question were the students attempting to answer with this investigation? b) Identify the independent variable and the dependent variable in the group’s investigation. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: Decontamination Process Decontamination Process (II DINQ1) A process (phytoremediation) has been developed that uses plants to remove contaminants from soils and water. Suppose a contaminated area (Brownfield site) in your town is being considered for this process. Identify at least three questions that would need to be answered before starting such a program. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: Enzymes2 (IV DINQ5) Enzymes 2 Science students conducted an investigation to determine how enzymes affect apple juice production. Procedure: 1. Place coffee filter in paper cone; cut off 2 cm of the bottom of the cone, leaving a small hole. 2. Place 30 mL of apple sauce into measuring cup, add 5 drops of enzyme A solution, and stir thoroughly. 3. Place a graduated cylinder under paper cone and add apple sauce to coffee filter, stirring every minute. 4. Measure volume of apple juice in cup after 5 minutes using graduated cylinder. 5. Repeat steps 1– 4 for a second trial. 6. Repeat steps 1–5 using enzyme B solution. 7. Repeat steps 1–5 using water. Amount of Juice Produced Enzyme Solution Trial 1 (ml) Trial 2 (ml) Average (ml) A 14 15 14.5 B 6 5 5.5 Water 5 5 5.0 a) Identify two variables that were held constant in the group’s experiment. b) Explain why it is important for these variables to be held constant. Write your answer in your answer booklet CAPT Science Open-­‐Ended Item: Stretching Experiment (II DINQ3) Stretching Experiment Students performed the following investigation. Procedure: 1. Cut a piece of plastic from each of the following: • dry-­‐cleaning bag • kitchen wrap • plastic sandwich bag • plastic grocery bag 2. Hold the sample of the dry-­‐cleaning bag between thumb and forefinger. 3. Attach a clamp to the bottom of the sample. 4. Add weights to the clamp and measure the length the plastic stretches. 5. Repeat for other samples. 6. Record data in table. The students recorded the following data from their investigation. Stretching Ability Plastic type Trial 1 dry-­‐cleaning bag 23 mm kitchen wrap 16 mm sandwich bag 14 mm grocery bag 7 mm a) After analyzing the data, the students concluded that the data supported their original hypothesis. What could have been the students’ hypothesis? b) Support your answer with specific information from the investigation. Write your answer in your answer booklet CAPT Science Open-­‐Ended Item: Energy Consumption (I DINQ 7) Energy Consumption The graph below shows the energy consumption for coal and natural gas in Connecticut for 40 years. a) Describe the overall trend for coal or natural gas from 1960–2000. b) Make a prediction regarding the energy consumption of coal or natural gas for the year 2015. c) Support your prediction with specific information from the graph. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: Yeast Experiment Yeast Experiment (V DINQ 9) A group of students wrote the following procedure for their experiment. Procedure: 1. Place 35 mL of 25% molasses solution into three small collection tubes. 2. Place 1 mL of the yeast suspension into each collection tube. 3. Place your palm over a small collection tube and mix each suspension well. 4. Carefully slide a larger tube down over the smaller tube. Invert the tube. Repeat for each tube. 5. Measure the height of the bubbles in the smaller tubes and record. 6. Place one tube at 10°C in a refrigerator. Leave the second one out at room temperature at 25°C. Place the third tube in a warming oven at 35°C. Make sure all tubes are in the dark and in an undisturbed location. Leave the three samples for 24 hours. 7. Measure the change in bubble size after 24 hours. Record the data. The table below shows the results of the group’s experiment. Temperature Height of CO2 Bubble (in °C) (in mm) 10 25 35 2 8 10 a) What conclusion can be drawn from the students’ experiment and results? b) Describe two ways the students could have improved their experimental design and/or validity of their results. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: Enzyme3 Investigation Enzyme3 Investigation (IV DINQ 2) A group of students hypothesized that adding an enzyme to applesauce would produce more juice than adding an enzyme to mashed pears. The students wrote the following procedure for their investigation. Procedure: 1. Place a coffee filter in each of two plastic funnels and place each funnel in a separate beaker. 2. Put 113 g of applesauce in one filter-­‐covered funnel. 3. Put 113 g of peeled, mashed pears in one filter-­‐covered funnel. 4. Add 3 drops of enzyme A to the applesauce and stir for one minute. 5. Add 3 drops of enzyme B to the mashed pears and stir for one minute. 6. Allow the fruit to sit for 10 minutes. 7. Measure and record the amount of juice contained in each beaker. 8. Repeat the procedure exactly for a second trial to verify data. The data collected during the investigation are shown in the table below. Type of Fruit Juice Produced (mL) Trial 1 12 13 Trial 2 11 12 Average 11.5 12.5 Applesauce Mashed Pears The students claimed that their original hypothesis was correct. a) Explain why the credibility of the students’ claim should be questioned. b) Describe two changes that the students should make to their procedure that would allow their original hypothesis to be more accurately tested and/or would ensure the accuracy of their results. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: Solar Cooker2 Investigation (I DINQ 9) Solar Cooker2 Investigation A student hypothesized that container size will affect the performance of solar cookers in heating water. The student wrote and performed the following procedure to support her claim. Procedure: 1. Line three identical cardboard boxes with aluminum foil to use as solar cookers. 2. Place the solar cookers outside in direct sunlight. 3. Place a large glass container of water in the center of the first box. 4. Record the initial temperature of the water. 5. Allow the container to sit in the sun for 2 hours, and then check and record the final temperature of the water. 6. Place a medium-­‐sized glass container of water in the center of the second box. 7. Repeat steps 4 and 5. 8. Place a small-­‐sized glass container of water in the center of the last box. 9. Repeat steps 4 and 5. The chart below shows the student’s data. Solar Cooker Data Container Temperature (°C) Initial Final Large 39 48 Medium 39 49 Small 39 49 a) Draw a conclusion regarding container size and the effectiveness of solar cookers in heating water, based on the student’s results. b) Describe two ways the student could have improved her experimental design and/or the validity of her results. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: Population Graphs for Taiwan Population Graphs for Taiwan (V DINQ 7) The graphs below show the population of Taiwan in 2000 and the predicted population of Taiwan in 2050. Use the graphs to: a) Draw a conclusion regarding the overall population change in Taiwan from 2000 to 2050. b) Describe two factors that may contribute to the predicted population changes from 2000 to 2050. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: Acid Rain Investigation 2 (II DINQ3) A group of students wrote the following procedure for their acid rain investigation. The chart below shows the students’ investigation results. Material Starting Ending Difference in Percent Mass (g) Mass (g) Mass (g) Loss Granite 6.2 6.2 0.0 0.0 Marble 8.7 8.1 0.6 6.9 Limestone 5.3 4.1 1.2 22.6 a) What was the problem the students were investigating? b) Describe two things the students could do to increase confidence in their results. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: Antifreeze Gene for Agricultural Crops (IV DINQ9) The DNA of Arctic flounder contains a gene that produces special antifreeze proteins that allow the fish to survive in cold Arctic waters. Geneticists have been able to isolate this gene and insert it into the DNA of certain agricultural crops. a) Explain how the antifreeze gene could be beneficial in growing agricultural crops, such as tomatoes. b) Describe two concerns a consumer might have regarding the insertion of animal DNA into agricultural crops. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: (II DINQ4) Polymer Investigation 3 A manufacturer claims that its kitchen wrap will stretch twice as much as the leading competitor’s plastic wrap without tearing. A group of students has gathered the following materials to test this claim. • one sample of kitchen wrap from the manufacturer making the claim • one sample of kitchen wrap from the leading competitor • masking tape • one clamp with a hook for adding weights • several weights • a metric ruler The students wrote the following procedure for their investigation. Describe at least three steps or pieces of information the students should add to the procedure to improve the design of their experiment. Write your answer in your answer booklet. CAPT Science Open-­‐Ended Item: Polymer Investigation 4 (II DINQ7) A student performed the following investigation to test four different polymer plastics for stretchability. Procedure: a. Take a sample of one type of plastic, and measure its length. b. Tape the top edge of the plastic sample to a table so that it is hanging freely down the side of the table. c. Attach a clamp to the bottom edge of the plastic sample. d. Add weights to the clamp and allow them to hang for five minutes. e. Remove the weights and clamp, and measure the length of the plastic types. f. Repeat the procedure exactly for the remaining three plastic samples. g. Perform a second trial (T2) exactly like the first trial (T1). The student recorded the following data from the investigation. a) Draw a conclusion based on the student’s data. b) Describe two ways the student could have improved the experimental design and/or validity of the results. CAPT Science Open-­‐Ended Item: Solar Cooker 3 ( I DINQ6) A group of students want to determine whether changing the color of the floor in a solar cooker will affect the rate at which food cooks. They use a pizza box to make a solar cooker. First, they cut a window in the box top, as shown below. They cover the flap with aluminum foil. The opening is covered with plastic wrap, which allows sunlight to enter the box. Several different colors of construction paper are obtained to line the floor of the box, each color to be used in a different trial. The finished solar cooker is shown below. a) Besides allowing sunlight through, what might be another purpose for covering the hole with plastic wrap? b) Identify the independent variable in this investigation. c) Explain why the flap on the top was covered with aluminum foil. CAPT RELEASED ITEMS OPEN ENDED RUBRICS: Rubric for Enzymes1 Possible Correct Responses: Conclusions: • Enzyme A produces the most apple juice from applesauce. • Enzyme B is not very effective at producing apple juice from applesauce. Reliability: • The results are reliable because the experimental design included a variable and a control. • The results are reliable because the experiment was repeated once and similar results were obtained. • The results should be questioned because only two trials were performed which is not sufficient data upon which to base a sound conclusion. 3-­‐Point Rubric: Score 3 The response draws a valid conclusion supported by the students’ experimental results and explains why the results were or were not reliable. Score 2 The response draws a valid conclusion supported by the students’ experimental results and provides a general explanation for why the results were or were not reliable (they did the experiment twice). -­‐or-­‐ The response explains in detail why the results were or were not reliable but fails to draw a valid conclusion supported by the students’ experimental results. Score 1 The response draws a valid conclusion supported by the students’ experimental results. The response may also indicate that the results were or were not reliable but fails to provide an acceptable explanation. -­‐or-­‐ The response indicates that the results were or were not reliable and provides a general explanation but fails to draw a valid conclusion supported by the students’ experimental results. Score 0 The response provides little or no accurate or relevant information. Strand IV: Cell Chemistry and Biotechnology Expected Performance: D INQ.7 Assess the reliability of the data that was generated in the investigation. Rubric for Genetically Modified Food Possible Correct Responses: • No data are presented to back up the statement(s). • “Similar survival rate” is vague; how similar was the survival rate? • Survival is only one aspect of health; no other potential side-­‐effects were mentioned (immune responses, tumor formation, etc.) • The conclusions were based on a three-­‐week study; what about long-­‐term consumption of GM grains? • There is no indication of who the researchers were affiliated with to determine whether they may have had an agenda/bias. • Two trials are not sufficient to draw a definitive conclusion. • Just because it does not affect mice does not mean it will not affect humans. • Other acceptable questions. 3-­‐Point Rubric: Score 3 The response provides three reasons a consumer should question the conclusions presented in the news release. Score 2 The response provides two reasons a consumer should question the conclusions presented in the news release. Score 1 The response provides one reason a consumer should question the conclusions presented in the news release. Score 0 The response describes little or no accurate or relevant information related to the credibility of the claims in the news release. Strand V: Genetics, Evolution and Biodiversity Expected Performance: D INQ.2 Read, interpret and examine the credibility and validity of scientific claims in different sources of information. Rubric for Acid Rain Possible Correct Responses: Needed Information: • You need to know how much vinegar was used in each container. • You need to know what type of vinegar was used in each container. • You need to know what materials to test. • You need to know what size/surface area of materials should be used. • You need to know how long each sample was rinsed in distilled water. • You need to know what drying method to use. • You need to know what size/type of container to use. • Other acceptable responses. 3-­‐Point Rubric: Score 3 The response describes three additional pieces of information that would be needed to accurately replicate the experiment. Score 2 The response describes two additional pieces of information that would be needed to accurately replicate the experiment. Score 1 The response describes one additional piece of information that would be needed to accurately replicate the experiment. Score 0 The response describes little or no accurate or relevant information from the acid rain investigation. Strand III: Global Interdependence Expected Performance: D INQ.4 Design and conduct appropriate types of scientific investigations to answer different questions. Rubric for Solar Cooker Investigation Possible Correct Responses: • Graduated cylinder: to accurately measure the water that must be added to the containers. • Three identical thermometers: to be suspended in the containers so that water temperature can be measured. • Timing device: to make sure the data for each container are collected within the same increments of time. • Safety equipment: glove or cloth pad to handle the containers safely after heating or safety glasses to protect eyes from hot water that could splash. • Lamp/light source/solar heat: to heat up the solar cooker. • Meter stick: to measure the same level of water for each container. • Other acceptable responses. 3-­‐Point Rubric: Score 3 The response identifies two pieces of equipment that the students would need in order to conduct their investigation and for each piece of equipment explains why it is necessary to the investigation. Score 2 The response identifies two pieces of equipment that the students would need in order to conduct their investigation and for one piece explains why it is necessary to the investigation. -­‐or-­‐ The response provides explanations for two pieces of equipment that are consistent with what is needed to conduct the investigation, but only identifies one piece of equipment or fails to identify either piece of equipment. Score 1 The response identifies two pieces of equipment that the students would need in order to conduct their investigation, but fails to correctly explain why either piece of equipment is necessary. -­‐ or-­‐ The response identifies one piece of equipment that the students would need in order to conduct their investigation and explains why it is necessary to the investigation. -­‐or-­‐ The student provides an explanation for a piece of equipment that is consistent with what is needed to conduct the investigation, but fails to identify the equipment. Score 0 The response identifies one piece of equipment that the students would need in order to conduct their investigation but fails to explain why it is necessary to the investigation. -­‐or-­‐ The response describes little or no accurate or relevant information related to the solar cooker investigation. Strand I: Energy Transformation Expected Performance: D INQ.6 Use appropriate tools and techniques to make observations and gather data. Rubric for Polymer Investigation Possible Correct Responses: Possible Question: • Which type of kitchen wrap is the strongest? • Is there a difference in strength between different kitchen wraps? • Which kitchen wrap can hold the most weight? • Other acceptable responses. Variables: The independent variable is the type of kitchen wrap used and the dependent variable is the amount of weight the kitchen wrap can hold without breaking. 3-­‐Point Rubric: Score 3 The response provides a scientifically valid question that could be answered using this procedure and identifies the independent and dependent variables. Score 2 The response provides a scientifically valid question that could be answered using this procedure, identifies the independent variable, but fails to identify the dependent variable. -­‐or-­‐ The response provides a scientifically valid question that could be answered using this procedure, fails to identify the independent variable, but correctly identifies the dependent variable. -­‐or-­‐ The response fails to provide a scientifically valid question that could be answered using this procedure, but correctly identifies the independent and dependent variables. Score 1 The response provides a scientifically valid question that could be answered using this procedure, but fails to identify the independent and dependent variables. -­‐or-­‐ The response fails to provide a scientifically valid question that could be answered using this procedure, correctly identifies the independent variable, but fails to identify the dependent variable. -­‐or-­‐ The response fails to provide a scientifically valid question that could be answered using this procedure, fails to identify the independent variable, but correctly identifies the dependent variable. Score 0 The response provides little or no accurate or relevant information related to the polymer investigation. Strand II: Chemical Structures and Properties Expected Performance: D INQ.5 Identify independent and dependent variables, including those that are kept constant and those used as controls. Rubric for Decontamination Process Possible Correct Responses: • What is/were the sources of contamination? • What are/were the contaminants of concern? • What is the extent of the affected property? • How deep does the contamination extend into the sediment? • What are the potential effects on the local ecosystem/food webs? • What type of ecological restoration is being sought (recreation, residential, etc.)? • What is the timeframe needed for total restoration? • What is the cost associated with this program compared to other programs? • What special resources (tools, people, etc.) are required? • Other acceptable questions. 3-­‐Point Rubric: Score 3 The response provides at least three relevant questions that would need to be answered before a town implements a phytoremediation program. Score 2 The response provides two questions that would need to be answered before a town implements a phytoremediation program. Score 1 The response provides one question that would need to be answered before a town implements a phytorernediation program. Score 0 The response provides little or no accurate or relevant information. Strand III: Global Interdependence Expected Performance: D INQ.1 Identify questions that can be answered through scientific investigation. Rubric for Enzymes2 Possible Correct Responses: • the amount of apple sauce used in measuring cup for each trial • the number of drops of each enzyme used in each trial (volume) • the stirring of the apple sauce (done every minute) • the amount of time enzyme is allowed to act before measuring apple juice • the cut off of 2 cm of the bottom of the cone allowing the apple juice to drain If any of these factors are not controlled, they could affect the results preventing the students from drawing a valid conclusion. If the students were to test more than one variable at a time, they would not be able to tell which variable is responsible for the results or if it was a combination of both. 3-­‐Point Rubric: Score 3 The response clearly identifies two specific variables that were held constant and provides an explanation that addresses the validity of the results in terms of testing too many variables at once. Score 2 The response clearly identifies two variables that were held constant but fails to provide a clear explanation of why it is important to hold these variables constant. -­‐or-­‐ The response clearly identifies one variable that was held constant and provides an explanation that addresses the validity of the results in terms of testing too many variables at once. Score 1 The response clearly identifies one variable that was held constant but fails to provide a clear explanation of why it is important to hold this variable constant. -­‐or-­‐ The response provides a clear explanation of why it is important to hold variables constant without clearly identifying any of the variables that were held constant. Score 0 The response provides little or no accurate or relevant information. Strand IV: Cell Chemistry and Biotechnology Expected Performance: D INQ.5 Identify independent and dependent variables, including those that are kept constant and those used as controls. Rubric for Stretching Experiment Possible Correct Responses: • The plastic used to make grocery bags is stronger (less likely to stretch or tear) than the other plastics: the plastic grocery bag stretched 7 mm, which was less than the other plastics. • The plastic used to make dry cleaning bags is weaker (more likely to stretch or tear) than the other plastics: the plastic dry cleaning bag stretched 23 mm, which was more than the other plastics. • The plastics used to make sandwich bags and grocery bags have similar strength: the amount of force required to stretch both of these plastics is similar, there was only a 2 mm difference. • Other acceptable hypotheses. 3-­‐Point Rubric: Score 3 The response provides a valid hypothesis that is supported by a description that includes specific information from the polymer investigation. Score 2 The response provides a valid hypothesis that is supported by a general description based on the polymer investigation (some of the plastics stretched more than others). Score 1 The response provides a valid hypothesis that is not supported by a valid general description based on the polymer investigation. Score 0 The response provides little or no accurate or relevant information. Strand II: Chemical Structures and Properties Expected Performance: D INQ.3 Formulate a testable hypothesis and demonstrate logical connections between the scientific concepts guiding the hypothesis and the design of the experiment. Rubric for Energy Consumption Possible Correct Responses: Trend: • Coal: coal use has varied over the 40-­‐year period, from very high in the 1960s to almost zero in the late 70s. Its use has been moderate since 1990. • Coal: coal use has declined overall from the beginning of the 40-­‐year period. • Natural Gas: natural gas use has increased relatively consistently since 1960. Coal Prediction: • The consumption of coal for 2015 will be approximately 40 trillion BTU. • The consumption of coal will stay level. • The consumption of coal for 2015 will be less than 40 trillion BTU. • The consumption of coal will continue to decline. • The consumption of coal for 2015 cannot be predicted with any degree of confidence. Support: • Coal consumption from 1990 to 2000 has been relatively stable at about 40 trillion BTU. • Coal use since 1990 peaked at 40 trillion BTU and has started to decline. • Coal use plummeted from almost 130 trillion BTU in 1965 to almost nothing from 1975 to 1980. Natural Gas Prediction: • The consumption of natural gas for 2015 will exceed 165 (or any number above that up to 250) trillion BTU. • Consumption of natural gas has increased from 30 trillion BTU in 1960 to over 160 trillion BTU in 2000 and there is no evidence that this trend will change. • The consumption of natural gas for 2015 will continue to increase but at a slower rate. Support: • Natural gas consumption has increased 20 to 30 trillion BTU for every 5 years that pass; therefore, it can be expected to continue increasing by this amount. • Consumption of natural gas has increased from 30 trillion BTU in 1960 to over 160 trillion BTU in 2000, and there is no evidence to suggest that this trend will change in the future. • From 1985 to 1995, consumption of natural gas increased by 30 trillion BTU for each 5 years that passed. From 1995 to 2000 it dropped off to a 20 trillion BTU increase. It can be expected to keep increasing, but at a slower rate. Note: Math errors aren’t penalized. 3-­‐Point Rubric: Score 3 The response describes the overall trend of either coal or natural gas and makes a valid prediction. The prediction is supported with specific information from the graph. Score 2 The response describes the overall trend of either coal or natural gas and makes a valid prediction but fails to provide sufficient support for the prediction. -­‐or-­‐ The response makes a valid prediction and supports the prediction with specific information from the graph but fails to accurately describe the overall trend of the resource. Score 1 The response describes the overall trend of either coal or natural gas but fails to make a reasonable prediction. -­‐or-­‐ The response makes a valid prediction but fails to provide sufficient support for the prediction and fails to describe the overall trend. Score 0 The response describes little or no accurate or relevant information. Strand I: Energy Transformation Expected Performance: D INQ.7 Assess the reliability of the data that was generated in the investigation. Rubric for Yeast Experiment Possible Correct Responses: Conclusions: • CO2 production in yeast increases with increasing temperature. • CO2 production in yeast decreases with decreasing temperature. Improvements: • The students could repeat the experiment to verify their results or do multiple trials at each temperature and average their results. • The students could add additional trials at higher (and/or lower) temperatures to see if the trend holds. • The students could perform the test again at smaller temperature increments. • The students could use a warming oven for the test tube subjected to room temperature (25°C), because in 24 hours, temperatures could fluctuate a few degrees which could affect results. • The students should use a consistent size for the gas collection tube (not clear in procedure). • Other acceptable responses. 3-­‐Point Rubric: Score 3 The response provides a valid conclusion and describes at least two ways the students could improve their experimental design and/or the validity of their results. Score 2 The response provides a valid conclusion and describes one way the students could improve their experimental design and/or the validity of their results. -­‐or-­‐ The response describes at least two ways the students could improve their experimental design and/or the validity of their results but fails to provide a valid conclusion. Score 1 The response provides a valid conclusion but fails to accurately describe a way the students could improve their experimental design and/or the validity of their results. -­‐or-­‐ The response describes one way the students could improve their experimental design and/or the validity of their results but fails to provide a valid conclusion. Score 0 The response describes little or no accurate or relevant information. Strand V: Genetics, Evolution and Biodiversity Expected Performance: D INQ.9 Articulate conclusions and explanations based on research data, and assess results based on the design of the investigation. RUBRIC FOR ENZYME3 INVESTIGATION Possible Correct Responses: Credibility Problem: • The procedure allowed for more than one variable. • The students used different enzymes in each type of fruit. • Data doesn’t support the claim. • Other acceptable responses Changes: • Use the same enzyme in each type of fruit (either A or B, but not both). • Use both enzymes on each fruit. • Add a control to the investigation (a sample of each fruit to which no enzyme is added). • Perform additional trials. • Other acceptable responses 3-­‐Point Rubric: Score 3 The response provides an explanation for why the credibility should be questioned and describes two changes the students could make that would allow their original hypothesis to be more accurately tested and/or would ensure the accuracy of their results. Score 2 The response provides an explanation for why the credibility should be questioned and describes one change the students could make that would allow their original hypothesis to be more accurately tested and/or would ensure the accuracy of their results. -­‐or-­‐ The response fails to provide or provides an incorrect explanation for why the credibility should be questioned, but describes two changes the students could make that would allow their original hypothesis to be more accurately tested and/or would ensure the accuracy of their results. Score 1 The response provides an explanation for why the credibility should be questioned, but fails to correctly describe any changes. -­‐or-­‐ The response fails to provide or provides an incorrect explanation for why the credibility should be questioned, but describes one change. Score 0 The response describes little or no accurate or relevant information related to the enzyme investigation. Strand IV: Cell Chemistry and Biotechnology Expected Performance: D INQ.2 Read, interpret, and examine the credibility and validity of scientific claims in different sources of information. Rubric for Solar Cooker2 Investigation Possible Correct Responses: Conclusion: • Container size appears to have little or no effect on the efficiency of solar cookers. • Small or medium-­‐sized containers heat water more than larger containers. • The investigation was not conclusive because the student failed to take temperature readings during the two hours, and while the final temperatures were similar, one container may have heated to that temperature faster than the others. • Other acceptable variations NOTE: If the student takes the position that the investigation results were inconclusive, the student must explain why. No credit will be awarded for simply stating that the investigation was not conclusive. Experimental Design Improvements: • Clearly indicate the amount of water that was put in each container, making sure it is identical. • Check and record the temperature at frequent and equal time increments during the investigation. • Perform additional trials; repeat the investigation exactly. • Add more containers of varying size to the investigation. • Test the three solar cookers at the same time. • Add a control (water in a container outside of the solar cookers). • Use an artificial light source because the sun’s rays vary due to clouds, etc. • Other acceptable responses 3-­‐Point Rubric: Score 3 The response provides a reasonable conclusion and describes two ways the student could have improved her experimental design. Score 2 The response provides a reasonable conclusion and describes one way the student could have improved her experimental design. -­‐or-­‐ The response describes two ways the student could have improved her experimental design, but fails to provide a reasonable conclusion. Score 1 The response provides a reasonable conclusion, but fails to describe how the student could have improved her experimental design. -­‐or-­‐ The response describes one way the student could have improved her experimental design, but fails to provide a reasonable conclusion. Score 0 The response describes little or no accurate or relevant information related to the solar cooker investigation. Strand I: Energy Transformations Expected Performance: D INQ.9 Articulate conclusions and explanations based on research data, and assess results based on the design of the investigation. Rubric for Population Graphs for Taiwan Possible Correct Responses: Conclusion: • The population in Taiwan will begin to stabilize over the next 50 years. • Population growth in Taiwan will slow down over the next 50 years. • There will be more older people and less younger people in 2050. • Other acceptable responses Factors: • Reproductive rates could decline some (people of reproductive age having fewer children). • Longevity could increase (increased survival rate for the elderly). • Mass immigration to other countries for various reasons. • Increased access to healthcare or better healthcare provisions. • Other acceptable responses 3-­‐Point Rubric: Score 3 The response draws a valid conclusion regarding the overall population change in Taiwan from 2000 to 2050 and describes two factors that may contribute to the predicted population changes from 2000 to 2050. Score 2 The response draws a valid conclusion regarding the overall population change in Taiwan from 2000 to 2050 and describes one factor that may contribute to the predicted population changes from 2000 to 2050. -­‐or-­‐ The response describes two factors that may contribute to the predicted population changes from 2000 to 2050, but fails to draw a valid conclusion regarding the overall population change. Score 1 The response draws a valid conclusion regarding the overall population change in Taiwan from 2000 to 2050, but fails to correctly describe any factors that may contribute to the predicted population changes from 2000 to 2050. -­‐or-­‐ The response describes one factor that may contribute to the predicted population changes from 2000 to 2050, but fails to draw a valid conclusion regarding the overall population change. Score 0 The response provides little or no correct information regarding population changes in Taiwan over the 50-­‐year period. Strand V: Genetics, Evolution and Biodiversity Expected Performance: D INQ.7 Assess the reliability of the data that was generated in the investigation. Rubric for Acid Rain Investigation 2 Possible Correct Responses: Possible Problem: • How does acid rain affect the mass of different building materials? • What type of building material holds up best in acid rain conditions? • What is the best type of building material to use outdoors? • What type of building material will degrade most quickly in acid rain conditions? • Other reasonable questions/problems Increase Confidence: • perform additional trials using the same building materials • add a control to their investigation • perform additional trials using water with different acidities • use samples that have the same surface area/same amount of material • use samples that have the same starting mass • let the samples sit in vinegar for a longer amount of time • make sure the temperature of the solvent is held constant for each sample • other acceptable responses 3-­‐Point Rubric: Score 3 The response describes a problem that could be investigated based on the procedure and data and describes two things the students could do to increase confidence in their results. Score 2 The response provides a problem that could be investigated based on the procedure and data and describes one thing the students could do to increase confidence in their results. -­‐or-­‐ The response describes two things the students could do to increase confidence in their results, but fails to or incorrectly describes a problem. Score 1 The response describes a problem that could be investigated based on the procedure and data, but fails to or incorrectly describes two things the students could do to increase confidence in their results. -­‐or-­‐ The response describes one thing the students could do to increase confidence in their results, but fails to or incorrectly describes a problem. Score 0 The response provides little or no correct information. Strand III: Global Interdependence Expected Performance: D INQ.3 Formulate a testable hypothesis and demonstrate logical connections between the scientific concepts guiding the hypothesis and the design of the experiment. Rubric for Antifreeze Gene for Agricultural Crops Possible Correct Responses: Benefits: · It could allow certain varieties of agricultural crops to be grown in colder areas where they were previously unable to be planted. · It could extend the growing season in cold areas for crops that contain the gene, allowing for a longer/larger harvest. It could reduce crop loss from early or late frosts. · Other acceptable responses Concerns: · Some crops may become allergenic. · The crops may cause unknown health risks. · The flavor of the crops may be compromised. · There may be unintended effects on the plants. · Labeling is not required for GM foods and vegetarians wouldn’t know whether they were consuming a product that includes animal DNA or not. · Genetic pollution (pollen from GM crops may cross-­‐pollinate non-­‐GM crops and/or organic crops, rendering farmers unable to certify their crops as organic). · Reduced genetic variation (farmers may opt to plant only the GM varieties to reap the benefits of the new genes). Crops may indirectly promote antibiotic resistance. (In transplanting genes, there is need for a marker to identify which cells have taken up the foreign gene. One way is to attach a gene for antibiotic resistance.) · Other acceptable responses 3-­‐Point Rubric: Score 3 The response explains how the antifreeze gene could be beneficial to certain agricultural crops and describes two concerns a consumer might have regarding the insertion of animal DNA into agricultural crops. Score 2 The response explains how the antifreeze gene could be beneficial to certain agricultural crops and describes one concern a consumer might have regarding the insertion of animal DNA into agricultural crops. or-­‐ The response describes two concerns a consumer might have regarding the insertion of animal DNA into agricultural crops, but fails to explain or incorrectly explains how the antifreeze gene could be beneficial to certain agricultural crops. Score 1 The response explains how the antifreeze gene could be beneficial to certain agricultural crops, but fails to describe or incorrectly describes concerns a consumer might have regarding the insertion of animal DNA into agricultural crops. -­‐or-­‐ The response describes one concern a consumer might have regarding the insertion of animal DNA into agricultural crops, but fails to explain or incorrectly explains how the antifreeze gene could be beneficial to certain agricultural crops. Score 0 The response provides little or no accurate or relevant information related to the tasks. Strand IV: Cell Chemistry and Biotechnology Expected Performance: D INQ.9 Articulate conclusions and explanations based on research data, and assess results based on the design of the investigation. Rubric for Polymer Investigation 3 Possible Correct Responses: Conclusion: • make sure each sample of plastic is exactly the same size • make sure the exact same length of plastic was left free-­‐hanging • indicate the mass of each weight added to the clamp • indicate how much time there was between each added weight (how long was each weight allowed to dangle before the next one was added) • measure the plastic after each weight is added to the clamp • indicate the total amount of weight each clamp held before the plastic samples began to tear • apply the weights with the same amount of force • perform multiple trials • other acceptable responses 3-­‐Point Rubric: Score 3 The response describes three steps or pieces of information the students should add to the procedure to improve the validity of the investigation. Score 2 The response describes two steps or pieces of information the students should add to the procedure to improve the validity of the investigation. Score 1 The response describes one step or piece of information the students should add to the procedure to improve the validity of the investigation. Score 0 The response provides little or no correct information. Strand II: Chemical Structures and Properties Expected Performance: D INQ.4 Design and conduct appropriate types of scientific investigations to answer different questions. Rubric for Polymer Investigation 4 Sample Response: Conclusions: · Plastic sample B has more stretchability than the other polymer plastics. · Plastic sample A has the least amount of stretchability compared to the other polymer plastics. · Not all polymer plastics have the same stretchability. · Different polymer plastics have different stretchability (and are therefore suited for different applications). · A reasonable conclusion cannot be drawn due to procedural errors. · Other reasonable conclusions Experimental Design Improvements: · Provide the before and after measurements for length (Did the samples all start out the same size?). · Make sure the samples are all of the same thickness. Variations in thickness could have caused variations in stretchability. · Perform additional trials. Some of the samples have similar stretchability (A and C, B and D). Two trials may not be enough to conclusively state that one is more stretchable than the other. · Indicate how many weights were added to the clamps (Was it the same number for each sample?). · Other acceptable responses 3-­‐Point Rubric: Score 3 The response draws a valid conclusion supported by the student’s data and describes two ways the student could have improved the experimental design and/or the validity of the results. Score 2 The response draws a valid conclusion supported by the student’s data and describes one way the student could have improved the experimental design and/or the validity of the results. -­‐ or-­‐ The response describes two ways the student could have improved the experimental design and/or the validity of the results but fails to draw or incorrectly draws a conclusion from the student’s data. Score 1 The response draws a valid conclusion supported by the student’s data but fails to describe, or incorrectly describes, how the student could have improved the experimental design and/or the validity of the results. -­‐or-­‐ The response describes one way the student could have improved the experimental design and/or the validity of the results but fails to draw or incorrectly draws a conclusion from the student’s data. Score 0 The response provides little or no correct information from the polymer investigation. Strand II: Chemical Structures and Properties Expected Performance: D INQ.7 Assess the reliability of the data that was generated in the investigation. Rubric for Solar Cooker 3 Sample Response: a) · The flap is covered with aluminum foil to reflect* the sunlight/heat into the box and warm the food in the solar cooker. OR · The foil helps make the box hotter. *unacceptable words: attract, draw, conduct, absorb, radiate b) · · The plastic wrap insulates the box. The plastic wrap prevents the warmed air inside the box from escaping. OR c) · 3-­‐Point Rubric: The colors of the box floor/the construction paper sheets are the independent variable. Score 3 The response provides a valid explanation for why the flap is covered with aluminum foil, why the box opening is covered with plastic wrap, and correctly identifies the independent variable in the investigation. Score 2 The response provides a valid explanation for why the flap is covered with aluminum foil and why the box opening is covered with plastic wrap but fails to identify, or incorrectly identifies, the independent variable. -­‐or-­‐ The response provides a valid explanation for why the flap is covered with aluminum foil or why the box opening is covered with plastic wrap and correctly identifies the independent variable in the investigation. Score 1 The response provides a valid explanation for why the flap is covered with aluminum foil or why the box opening is covered with plastic wrap and fails to identify, or incorrectly identifies, the independent variable. -­‐or-­‐ The response correctly identifies the independent variable but fails to explain, or incorrectly explains, why the flap is covered with aluminum foil and why the box opening is covered with plastic wrap. Score 0 The response provides little or no correct information. Strand I: Energy Transformations Expected Performance: D INQ.6 Use appropriate tools and techniques to make observations and gather data