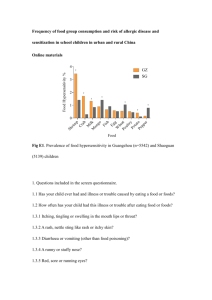
bean lightest of mass average bean of mass average mass = relative
advertisement

CHEMISTRY/EXPERIMENT COUNTING PARTICLES & RELATIVE MASS Counting a large number of items is a time consuming, tedious task. One way to minimize the difficulty of such a job is to use units larger than "one" in the counting process. It's a lot easier to count 15 dozen eggs than it is to count 180 eggs. In chemistry, there is a system for counting atoms and molecules, which is somewhat similar. To become familiar with the system, complete this experiment involving different kinds of beans. In the experiment, beans will be counted in groups called "bunches". A bunch is a counting number, similar to a dozen or couple. The number of beans in a bunch can be found by relating it to an arbitrary standard of mass. This procedure will avoid the burden of actually counting a large number of beans. In the second part of the experiment a bunch of beans is related to a mole of atoms or molecules. PROCEDURE: 1. Place a weigh boat on the balance. “Re-zero” the balance (press tare). 2. Count out exactly 100 beans of one type, discarding any beans which differ greatly from an average bean. 3. Place the 100 beans in the weigh boat to obtain the mass of 100 beans. 4. Repeat this procedure for each bean type provided. Record values. 5. Calculate the average mass of one bean of each type. Record values. 6. Using the formula provided below, calculate the relative mass of each type of bean by comparing it to the lightest bean. Show your calculations in the space provided below and record your values in the table. relative mass = average mass of bean average mass of lightest bean 7. Place beans one-by-one in the weigh boat until you reach the relative mass for that bean type. 8. Count the number of beans in step 7. Record this number in the table. Done with care, all the numbers should be about the same value. CHEMISTRY/EXPERIMENT COUNTING PARTICLES & RELATIVE MASS DATA: Bean Type white mass of 100 beans Calculated average mass Name ______________________ Date ________________ Per ___ pinto lentil kidney g g g g g g g g Calculated relative mass # of beans in relative mass = “bunch” Conversion units: 1 “bunch” of white beans = ______ beans = _____g 1 “bunch” of pinto beans = ______ beans = _____g 1 “bunch” of lentil beans = ______ beans = _____g 1 “bunch” of kidney beans = ______ beans = _____g QUESTIONS / PROBLEMS: SHOW FACTOR-LABEL METHOD FOR #3-6. 1. How many beans are in a “bunch”? 2. The amount of beans in a bunch is the always the same. However, the relative mass is different. Why is this so? 3. How many beans would you have in 15 bunches of black beans? (You didn’t use black beans. However, you can do this calculation if you refer back to question 1) 4. How many bunches would you have if you had 10.0 grams of the white bean? 5. What is the mass of 35 bunches of the heaviest bean? (Use the relative mass for the heaviest beans as the conversion between bunches and grams.) 6. In chemistry we use a unit called the mole to represent a particular number of particles. This is analogous to “bunches” in this lab. One mole is equivalent to 6.02 x 1023 particles. a) If you had 1 mole of garbanzo beans how many beans would you have? b) If you had 1 mole of Helium atoms how many atoms would you have? c) If you had 1 mole of anything how much would you have?