Thermodynamic Exploration of Eosin-Lysozyme
advertisement

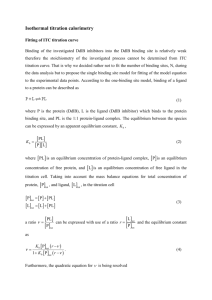
Thermodynamic Exploration of Eosin-Lysozyme Binding A Physical Chemistry and Biochemistry Laboratory Experiment Andrew J. Huisman, Lydia R. Hartsell, Brent P. Krueger*, and Michael J. Pikaart* Hope College, Department of Chemistry, Holland MI 49423 Abstract We have developed a modular pair of laboratory experiments for use both in undergraduate physical chemistry and biochemistry. Both laboratories examine the thermodynamics of the binding of a small molecule, eosin, to the protein lysozyme. The assay for binding is the quenching of lysozyme fluorescence by eosin through resonant energy transfer. In both experiments students measure fluorescence quenching at constant lysozyme concentration as a function of added eosin and determine the dissociation constant and ∆G for binding. In the physical chemistry experiment, students repeat the fluorescence measurements at several temperatures to determine the temperature-dependence of ∆G, and therefore ∆H and ∆S as well. Typical student results yield a dissociation constant, KD, of 22.7 ± 2.0 -1 µM and a binding ∆G of -26.49 ± 0.22 kJ mol at 25 °C. ∆H and ∆S of binding are found to be -4.20 ± -1 -1 -1 0.80 kJ mol and 74.6 ± 2.7 J mol K , respectively. This pair of laboratories represents an interdisciplinary module in which students in different courses examine the same system, but from different perspectives and with different emphases. Keywords: Upper-Division Undergraduate, Biochemistry, Physical Chemistry, Laboratory Instruction, Collaborative/Cooperative Learning, Communication / Writing, Hands-on Learning, Fluorescence Spectroscopy, Proteins / Peptides, Spectroscopy, Thermodynamics 1 Thermodynamic Exploration of Eosin-Lysozyme Binding A Physical Chemistry and Biochemistry Laboratory Experiment Andrew J. Huisman, Lydia R. Hartsell, Brent P. Krueger*, and Michael J. Pikaart* Hope College, Department of Chemistry, Holland MI 49423 Fluorescence spectroscopy has become a common tool for examining molecules of biological interest such as proteins due to the intrinsic fluorophoric nature of tryptophan and tyrosine residues and the relative ease of site-specific fluorescent labeling. In recent years, there has been a resurgence in the use of fluorescence with examples such as viscosity measurements1, single-molecule measurements2,3, inclusion complexes4, and protein-ligand binding5,6 appearing in this journal alone. Because of its sensitivity and versatility it is likely that fluorescence will continue to grow in importance as a spectroscopic technique for biological systems7. The purpose of this article is to describe an experiment that uses a fluorescence assay to measure the extent of protein-substrate binding at several temperatures. The temperature dependence of KD, the dissociation constant, leads to quantification of ΔG, ΔH, and ΔS for the lysozyme-eosin binding event. This exercise was developed as a modular laboratory to be used both in teaching physical chemistry principles using biological systems and for teaching biochemistry using spectroscopic methods. Students will learn and apply analytical, spectroscopic, and biochemical skills as well as analysis techniques specific to spectroscopy and biochemistry. This experiment is appropriate for junior or senior level chemistry, biochemistry or biology majors, and was designed jointly for our Chem 345 Physical Chemistry I Laboratory and our Chem 315 Biochemistry Laboratory. The biochemistry variant of the lab involves measuring KD at one temperature and can be completed in one 3-hour period. The physical chemistry variant, which includes a more complete thermodynamic analysis, requires two 3-hour periods. The pair of labs represents an interdisciplinary module in which students in both courses see similar techniques applied to the same system from different perspectives and emphases. 2 Theory Lysozyme is a well characterized protein that is often used to benchmark equipment and is commercially available. It was discovered by Alexander Fleming, who noticed that an inadvertent drop of nasal mucous on a bacteriological plate prevented bacterial growth 8. Lysozyme is also present in bird egg-white, likely to ward off bacterial growth before chick hatching. Enzymatically, lysozyme is an endoglycosidase with specificity for the (N-acetyl glucosamine – N-acetylmuramic acid)n polysaccharide that makes up the structural scaffold of the bacterial cell wall. David Phillips solved the X-ray crystal structure of hen egg-white lysozyme (HEWL)9, the first enzyme structure to be determined, and subsequently deduced the generally accepted “Phillips mechanism” of lysozyme activity. This molecule has since proven to be paradigmatic regarding enzyme-substrate interactions and protein structure and folding. Although not biologically important, HEWL forms a complex with the fluorescent dye eosin, a tetrabrominated derivative of fluorescein. Eosin can enter the protein’s hydrophobic interior to form a complex with 1:1 stoichiometry10-12 and perturb the protein’s intrinsic fluorescence. HEWL fluoresces in , the near UV with an emission maximum near 340 nm due to the presence of six tryptophan residues. Eosin absorbs weakly in the near UV, sufficiently overlapping with the fluorescence of HEWL to quench its emission via resonant energy transfer (RET) 7,13,14. Thus, a decrease in fluorescence emission from HEWL is an easily observable assay of lysozyme-eosin complexation. In general, the binding of a ligand L to a protein P is schematically rendered as P + L ⇔ PL, where PL is the complex formed after binding. Physical chemists generally quantify the forward equilibrium (or binding) constant KB = [PL] . Biochemists, by convention, use KD, the reverse [P][L] equilibrium (or dissociation) constant, as a measure of binding affinity. KD = [P] [L] [PL] (1) 3 KD is often measured through the fractional saturation of ligand binding to protein, i.e. the ratio of the amount of protein containing bound ligand, [PL], to the total amount of protein in the reaction mix, [P]tot. Assuming that all of the protein is either free in solution, or complexed with the desired ligand such that [P]tot= [P] + [PL], and substituting into Eq. 1 yields [ PL] = [ L] [Ptot ] [ L] + K D (2) This function defines a rectangular hyperbola with an asymptote representing protein fully occupied (i.e. ! saturated) by ligand when [L] >> KD. The simplest treatment of binding approximates the free ligand concentration [L] as equal to the total ligand concentration [Ltot] (i.e. [ L] " [ Ltot ] ). This is the case, for example, when the protein is at a very low concentration, allowing an effective excess of available ligand at all measured concentrations, or also when the volume of added!ligand is much greater than that of the binding reaction. For instance, the latter situation applies when measuring binding to membrane bound or intracellular receptors, where the proteins are not free to diffuse through the entire volume available to the ligand. KD can thus be determined by following the concentration of complex formed as a function of added L either by directly fitting the hyperbolic equation using non-linear regression analysis or graphically by using the linearly transformed form of Equation 2 as in traditional Scatchard analysis (e.g. [ PL] = "K D [PL] + P ). [ tot ] [L] The current experiment involves freely diffusing proteins in a small volume with ligand. It is ! expected that a significant fraction of the ligand added to the protein (especially at lower concentrations of ligand) will become bound as PL complex, a condition referred to as ligand depletion. This requires a more rigorous approach in analyzing these data, which is also more instructive for students. Substituting [L] = [L]tot - [PL] and [P] = [P]tot - [PL] into Equation 1 yields KD = ([P]tot " [PL])([L]tot " [PL] [PL] (3) which can be rearranged as quadratic in [PL] and solved to give ! 4 [PL] = ([P]tot + [L]tot + K D ) " ([P]tot + [L]tot + K D ) 2 " 4([P]tot [L]tot ) 2 (4) In the experiment described here, we take advantage of the fact that free protein exhibits a ! fluorescence intensity, I0, that is much larger than that of the complex, IC. For given concentrations of protein and complex, the total observed fluorescence, [P]totIOBS, is simply the sum of the total fluorescence from free protein, [P]I0, and the total fluorescence from protein bound with ligand, [PL]IC [P ] tot IObs = [P ]I0 + [ PL]IC (5) This is valid provided that there are really only two possible emissive states of lysozyme, i.e. free or with eosin bound in!a single, well-defined way, which is reasonable in this case. Substituting [ P ] = [ P ] tot " [ PL] and rearranging gives [ PL] [P ] tot I "I #I = 0 Obs = Obs I0 " IC #ISat ! (6) where ΔIObs is the observed quenching at the particular ligand concentration and ΔISat is the maximum quenching observed at saturating ligand concentrations. ! Substituting Eq. 6 into Eq. 4 yields "Iobs = "Isat ([ P ] tot + [ L] tot + K D ) # ([ P ] tot + [ L] tot + K D ) 2 # 4 [ P ] tot [ L] tot 2[ P ] tot [ ] (7) ! an equation in which all parameters other than KD are either known ([P]tot and [L]tot) or measured (ΔISat and ΔIObs). Thus, Eq. 7 may be employed to determine KD via nonlinear least squares fitting. Finally, using the relation "G = ! RT ln K B = ! RT ln 1 = RT ln K D KD (8) the Gibbs Free Energy for binding can be determined at a given temperature from a measured KD. Making such a measurement at several temperatures shows the experimental temperature dependence of ΔG, from which the fundamental thermodynamic quantities ΔH and ΔS may determined via a linear fit to the equation !G = !H-T!S . 5 In this experiment, students learn through instruction and hands-on experience the operation of a UV-Vis spectrophotometer and a fluorometer, preparation of solutions of micromolar lysozyme and eosin using micropipettes, characterization of solutions by extinction-coefficient measurements, and – in the physical chemistry version – acquisition of fluorescence spectra with temperature control. Note that, to our knowledge, these are the first reported values of the ∆H and ∆S of the lysozyme-eosin binding event. Experimental Procedure Details of the two experimental procedures are given in the supplementary materials and are only briefly summarized here. The data presented in Figures 1-3 are typical student results acquired by one physical chemistry lab group during their standard coursework. Students are provided with solutions of HEWL, eosin, and Tris/NaCl buffer (pH 8.2). HEWL was either purified from egg-white as part of an earlier experiment (biochemistry lab) or obtained commercially from Sigma (L-6876) (physical lab). The precise concentration of the lysozyme solution is measured as -1 -1 part of the lab using an extinction coefficient of 37,789 M cm at 280 nm 15. Eosin was obtained as a solution in ethanol from Sigma (HT110-1-16), nominally 7.7 mM. Students determine the exact concentration of the eosin solution by diluting 50-fold in basic ethanol (containing roughly 0.01 M NaOH) -1 -1 and measuring the absorbance using an extinction coefficient of 112,000 M cm at 526 nm 16. Students prepare and characterize solutions with a constant amount of lysozyme (approx. 3 µM) and varying concentrations of eosin (0-50 µM) in UV-transparent 1 cm cuvettes. Fluorescence spectra are then recorded with an excitation wavelength of 280 nm and an emission window ranging from 300 – 420 nm. In the physical chemistry version, samples are stirred to aid rapid temperature changes and the spectra are repeated at several temperatures between 5 °C and 35 °C. Analysis is then conducted in a spreadsheet, converting fluorescence intensity data (Figure 1) into quenching data (Eq 6) and then fitting to Equation 7 (see Fig. 2) using the nonlinear least squares algorithm supplied through the Solver tool in Excel. The resulting value of KD is used to calculate ΔG via Equation 8. In the biochemistry lab, students also apply the quenching data to the inverse of Eq. 2 and obtain a comparative value of KD through a Scatchard plot. The Scatchard plot is modestly non-linear in 6 this case, revealing the presence of ligand depletion at low [L] (data not shown). In the physical chemistry variant students determine the uncertainty in KD using the SolverAid tool 17, and continue with data from multiple temperatures to generate a linear fit of ΔG versus temperature, yielding ΔH and ΔS (Figure 3). As part of their analysis, students are asked to compare this mainly entropically driven binding event to other chemical processes. Results were obtained on a Aminco Bowman Series 2 Luminescence Spectrometer using a recirculating water bath to control the temperature and a SPEX Fluorolog-2 using a Peltier temperature controller from Quantum Northwest Labs. Either a Shimadzu UV-1601PC or a Cary 5E UV/Vis spectrophotometer was used for absorption measurements. Supplementary Materials The supplementary materials contain the biochemistry and physical chemistry student handouts, instructor/TA notes, a template spreadsheet for the fitting and Scatchard analysis in biochemistry, and a template spreadsheet for the complete physical chemistry (temperature-dependent) analysis. Note that the physical chemistry template spreadsheet does not include the SolverAid macro needed to determine the uncertainty in Kd. This may be downloaded as part of the MacroBundle package from http://www.bowdoin.edu/~rdelevie/excellaneous/. Hazards Eosin Y is harmful if swallowed and is an irritant to eyes, skin, and mucus membranes. It is a brominated molecule and should be handled with care. Students should wear gloves and safety glasses while working with the eosin solutions. Lysozyme and Tris/NaCl buffer should be handled with the care afforded to any other chemical, but are not uniquely hazardous. Acknowledgements The authors are grateful to Drs. Karen and Mark Muyskens of Calvin College for use of their fluorometer during development of this laboratory and to the Mayo Clinic for donation of their fluorometer for ongoing use. AJH and BPK acknowledge support from the Research Corporation Cottrell College 7 Science Awards. BPK was also supported by the Towsley Foundation. MJP was supported through the NIH-AREA program. Finally, we thank physical chemistry students Kurt Blohm and Emily Campbell for acquiring the data presented here. References (1) (2) (3) (4) Windisch, C. F., Jr.; Exarhos, G. J.; Sharma, S. K. J. Chem. Educ. 2005, 82, 916-918. Harbron, E. J.; Barbara, P. F. J. Chem. Educ. 2002, 79, 211-213. Zimmermann, J.; Dorp, A. v.; Renn, A. J. Chem. Educ. 2004, 81, 553-557. Crane, N. J.; Mayrhofer, R. C.; Betts, T. A.; Baker, G. A. J. Chem. Educ. 2002, 79, 1261- (5) (6) Marcoline, A. T.; Elgren, T. E. J. Chem. Educ. 1998, 75, 1622-1623. Williams, K. R.; Adhyaru, B.; Pierce, R.; Schulman, S. G. J. Chem. Educ. 2002, 79, 115- 1263. 116. (7) Lakowicz, J. R. Principles of Fluorescence Spectroscopy; Kluwer Academic/Plenum Publishers: New York, 1999. (8) Fleming, A. Proc. Royal Soc. (London) 1922, 93, 306-319. (9) Blake, C. C.; Koenig, D. R.; Mair, G. A.; North, A. C.; Phillips, D. C.; Sarma, V. R. Nature 1965, 206. (10) Baugher, J. F.; Grosswei, L. I.; Lewis, C. J. Chem. Soc. Faraday II 1974, 70, 1389-1398. (11) Jordanides, X. J.; Lang, M. J.; Song, X.; Fleming, G. R. J. Phys. Chem. B 1999, 103, 7995-8005. (12) Kepka, A. G.; Grossweiner, L. I. Photochem. Photobiol. 1973, 18, 49-61. (13) Resonance Energy Transfer; Andrews, D. L.; Demidov, A. A., Eds.; John Wiley & Sons: New York, 1999. (14) Förster, T. Ann. Physik 1948, 2, 55-75. (15) Gill, S. C.; von Hippel, P. H. Anal. Biochem. 1989, 182, 319-326. (16) Seybold, P. G.; Gouterman, M.; Callis, J. Photochem. Photobiol. 1969, 9, 229-242. (17) de Levie, R. Advanced Excel for scientific data analysis; Oxford University Press: New York, 2004. 8 Figure 1. Fluorescence spectra of samples with equimolar lysozyme and increasing eosin concentrations. The lysozyme concentration is approximately 3 µM and the eosin concentration ranges from 0 to 50 µM as indicated. Excitation was at 280 nm and the samples were maintained at 25 °C. These data were acquired by one group during our physical chemistry laboratory course in the fall 2005 semester. 9 Figure 2. A typical nonlinear least squares fit of Equation 7 to quenching data. These are the same data shown in Figure 1 and result in a KD of 22.7 ± 2.0 µM. 10 -1 - Figure 3. A linear fit of ΔG as a function of temperature. These results yielded ΔS = 74.6 ± 2.7 J mol K 1 -1 -1 and ΔH = -4.20 ± 0.80 kJ mol . ΔG at 25 °C is -26.49 ± 0.22 kJ mol , corresponding to the data shown in Figures 1 and 2. 11