Bohr Models Worksheet
advertisement
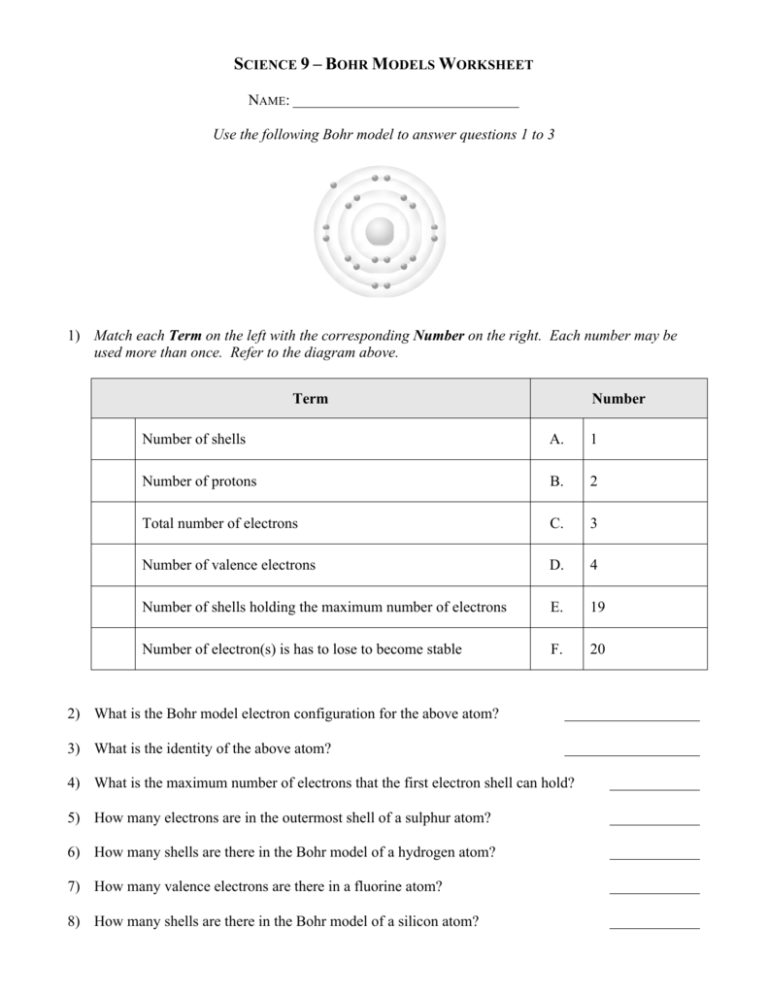
Use with textbook pages 64–67. Use the periodic table on answer questions 8 to 12. The periodic table and SCIENCE 9 – BOHR MODELS WORKSHEET atomic theory 8. How many electrons are shell of a sulphur (S) ato NAME: A. 1 Use the following Bohr model to answer questions 1 to 6. Use the following Bohr model to answer questions 1 to 3 B. 2 C. 6 D. 7 9. How many electrons are shell of a fluorine (F) ion A. 1 B. 2 1) Match each Term on the left with the corresponding Number on the right. Each number C. 7 may be Match the Term on the left with the corresponding used more than once. Refer to the diagram above. Number on the right. Each Number may be used D. 8 more than once. Refer to the diagram above. 10.Number How many shells are the Term Term Number model of an aluminum (A Number of shells 1. number of A. 0 shells B. 1 2. number of C. 2 Number of protons protons D. 3 total number of E. 4 Total number3.of electrons electrons F. 19 4. number of G. 20 Number of valence electrons valence electrons 5. number of Number of shells holding itthe maximum electron(s) has to lose number of electrons to become stable Number of electron(s) is has tooflose to become stable 6. number shells holding the maximum number of 2) What is the Bohr modelelectrons electron configuration for the above atom? A. 1 A. 1 B. 2 C. 3 D. 4 B. 2 C. 3 D. 11.4 Which of the following r model electron arrangem (Cl) atom? E. 19 F. 20B. 2, 2, 13 A. 2, 15 C. 2, 8, 7 D. 2, 8, 8 12. What do a beryllium (Be (Ne) atom have in comm 3) What is the identity of the above atom? Circle the letter of the best answer. 4) What is the maximum7.number that thenumber first electron shell can hold? What of is electrons the maximum of electrons A. They have full outer s that the first electron shell can hold? B. They have the same n 6) How many shells are there in the Bohr model of a hydrogen atom? A. 1 C. They have the same n shells. 7) How many valence electrons are there in a fluorine atom? D. None of the above 5) How many electrons are in the outermost shell of a sulphur atom? B. 2 C. 4 8) How many shells are there D. in 8 the Bohr model of a silicon atom? © 2007 McGraw-Hill Ryerson Limited Section 2.3 The Periodic Table and Atomi (c) number of electrons (e) Bohr model of a nitrogen atom (d) number of valence electrons (e) Bohr model of (a) model number of protons 9) Fill diagram. 2. in the blanks beside each Bohr a)2. C C (b) (a) (c) (b) (d) (c) (e) (d) a nitrogen atom number of shells number of protons number of electrons number of shells number of valence electrons number of electrons Bohr model of a number of valence electrons (e) Bohr model of a (a) number of protons b)3. (b) number of shells (a) number of protons (c) number of electrons (b) number of shells O (d) number of valence electrons (c) number of electrons (e) Bohr model of an 10) Draw Bohr diagrams for the following. Make sure you show the correct number of neutrons (d) number of valence electrons 3. O found in the nucleus. Write the electron configuration for each Bohr diagram. (e) number Bohr model of an (a) of protons 27 4. (a) 39K (b) Al (b) number of shells Ne 4. (a) number of protons (c) number of electrons (b) number of shells (d) number of valence electrons Ne (c) number of electrons (e) Bohr model of a (d) number of valence electrons 5. The four elements above are inmodel the same (e) Bohr of a period. What do you notice about the number of shells for elements belonging to the same period? 5. The four elements above are in the same period. What do you notice about the number of shells for elements belonging to the same period? (c) 11B (d) 7Li 36 MHR • Section 2.3 The Periodic Table and Atomic Theory 36 MHR • Section 2.3 The Periodic Table and Atomic Theory © 2007 McGraw-Hill Ryerson L © 2007 McGraw-Hill Ryerson





