Structure
Determination:
Mass Spectrometry,
IR, and UV Spectroscopy
219
Solutions to Problems
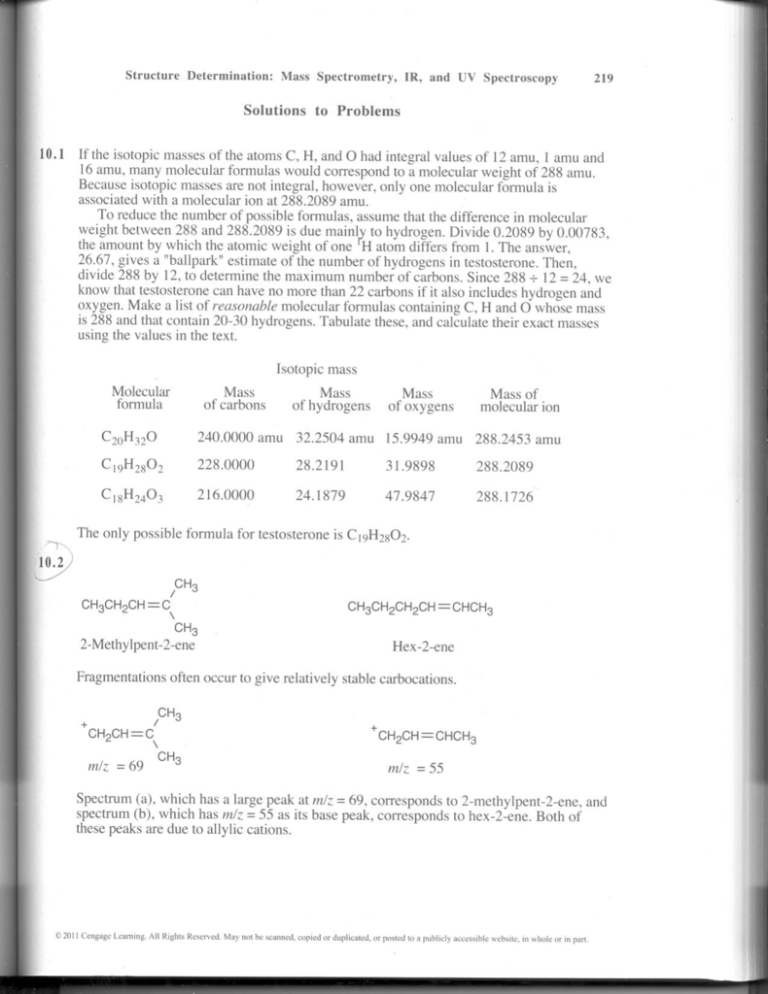
10.1 If the isotopic masses of the atoms C, H, and ° had integral values of 12 amu, 1 amu and
16 amu, many molecular formulas would correspond to a molecular weight of 288 amu.
Because isotopic masses are not integral, however, only one molecular formula is
associatedwith a molecular ion at 288.2089 amu.
To reduce the number of possible formulas, assume that the difference in molecular
weight between 288 and 288.2089 is due mainlf.'to hydrogen. Divide 0.2089 by 0.00783,
the amount by which the atomic weight of one H atom differs from 1.The answer,
26.67, gives a "ballpark" estimate of the number of hydrogens in testosterone. Then,
divide 288 by 12, to determine the maximum number of carbons. Since 288 + 12 =24, we
know that testosterone can have no more than 22 carbons if it also includes hydrogen and
oxygen. Make a list of reasonable molecular formulas containingC, Hand ° whose mass
is 288 and that contain 20-30 hydrogens. Tabulate these, and calculatetheir exact masses
using the values in the text.
Isotopic mass
Molecular
formula
9
Mass
of carbons
Mass
of hydrogens
Mass
of oxygens
Mass of
molecular ion
C2oH32O
240.0000 amu
32.2504 amu 15.9949 amu
288.2453 amu
C 19H2S02
228.0000
28.2191
31.9898
288.2089
CIsH2403
216.0000
24.1879
47.9847
288.1726
The only possible formula for testosterone is C19H2S02.
CH3CH2CH
=
I CH3
C
\
CH3
2-Methylpent-2-ene
Hex -2-ene
Fragmentationsoften occur to give relatively stable carbocations.
+
CH2CH=
m/z = 69
CH3
cl\
CH3
m/z = 55
Spectrum (a), which has a large peak atm/z= 69,corresponds to 2-methylpent-2-ene, and
spectrum (b), which has m/z = 55 as its base peak, corresponds to hex-2-ene. Both of
these peaks are due to allylic cations.
~ 2011 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated,
or posted to a publicly accessible website, in whole or in part.
.
220
Chapter
10
A((3) In a mass spectrum,the molecular ion is both a cation and a radical. When it fragments,
V
two kinds of cleavage can occur. (1) Cleavage can fomi a radical and a cation (the species
observed in themass spectrum). Alpha cleavage shows this type of pattern. (2) Cleavage
can form a neutral moleculeand a different radical cation (the species observed in the mass
spectrum).Alcohol dehydration and the McLaffertyrearrangement show this cleavage
pattern.
\
For each compound, calculatethe mass of the molecular ion and identifythe functional
gro~ps present. Draw the fragmentation products and calculate their masses.
1
(a)
+"
' 0
1/
/C,
l
.
: CH2CH2CH3
]
M+ =86
H3C
[ H3C-C=O
yC'
]
+" CH2CH2CH3
+"
,)1
H3:C
=43
mlz
.
0
l
+
Alpha
cleavage
I
_
Alpha
CH2CH2CH3
]
H3C". +
[ O=C-CH2CH2CH3 ]
+
mlz =71
In theory, alpha cleavage can take place on either side of the carbonyl group, to produce
M+
=86
CI"
eavage
=43 and mlz =71. In practice,
the cations with mlz
cleavage occurs on the more
substituted side of the carbonyl group, and the fIrst cation, with mlz =43, is observed.
+"
+"
(b) I
-
lUOH
[
]
DehYdratiOn.-O
J
M+= 100
+ H20
mlz = 82
Dehydration of cyclohexanolproduces a cation radical with mlz =82.
(c)
H,
+"
CH3 0
+" McLafferty..
I
1/
J rearrangement
[CH3CHCH2CCH3
M+ = 100
H H~
H,\ /
0
II
H3C_C~C'
/
C
CH
9~
H
?
'iC,
[ H2C
~
mlz
CH3] .
=58
+
1\
H
+"
H
?lH2
C
H3C/
'H
The cation radical fragment resulting from McLafferty rearrangementhas mlz =58.
I
(d)
+"
.
CH2CH3
,
[H3CtCH2
CH2CH3
Alpha
1
N,
CH"cH3
]
..
H3C" +
cleavage
[
'iN,
H"c
M+ = 101
mlz
Alpha cleavage of triethylamineyields a cation with mlz = 86.
iC 20 II Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated,
+
I
CH2CH3
=86
]
or posted to a publicly accessible website, in whole or in part.
9
.0.4
Structure
Determination:
Mass Spectrometry,
IR, and UV Spectroscopy
221
The molecule, 2-methylpentan-2-ol,produces fragments that result from both dehydration
and from alpha cleavage.Two different alpha cleavage products can be detected.
+.
CH
.
Dehydration
[CH3CH2CH2t~3 ]
M+
=102
!
Alpha
cleavage
+
OH
CH3CH2CH2<
[
+
CH3
mlz
/'"'
=87
H3C..
[
J
HO'?"CH3r
CH3 J
mlz = 59
0.5') We know that: (1) energy increases as wavelength decreases, and (2) the wavelength of Xradiation is shorter than the wavelength of infrared radiation. Thus, we estimate that an X
ray is of higher energy than an infrared ray.
J
E
=hv =he/A; h =6.62 X 10-34 J.s; c =3.00 X 108mls
for.A = 1 x 1O-{)m (infrared radiation):
E
= (6.62 X 10-34J.s) (3.00 X 108mls) = 2.0 X 10-19J
1.0 X 1O-{)m
for .A = 3.0 x 10-9 m (X radiation):
-34
E
= (6.62 x 10
)(
8
J.s 3.00 x 10 mls
3.0 x 10-9m
)
= 6.6 X 10-17J
ConfIrmingour estimate, the calculation shows that an X ray is of higher energy than
infrared radiation.
When comparing radiation expressedby different units, convert radiation in m to radiation
in Hz by the equation:
c
v
=A =
3.00 X 108 mls
9.0 X 1O-{)m
= 3.3 X 1013 Hz
E =hv shows that the greater the value of v, the greater the energy. Thus,
radiation with v =3.3 X 1013 Hz (A. =9.0 x 10-6m) is higher in energy than radiation with
The equation
v
=4.0 x 109 Hz.
<02011 Cengage Learning. All Rights Reseryed. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
222
Chapter
10.6
Use the expression for energy shown in this section. Convert frequency to wavelength
when you need to.
(a) E
10
= 1.20 X 10-4kJ/mol =
f...(in m)
(b)
1.20 x 10-4kJ/mol
5.0 x 10-11
= 2.4 X 106kJ/molfor a gammaray.
E = 4.0 X 104kJ/molfor an X ray.
(c) v
E
=
.£..;
f...
f...
= £ = 3.0x
108mIs
6.0 X 1015Hz
v
= 5.0 x 1O-8m
= 1.20 X 19-4kJ/mol = 2.4 x 103kJ/mol for ultraviolet light
5.0.'x 10-8
.
(d) E =2.8 X 102kJ/molforvisible light.
10.7
(e)
E =6.0 kJ/mol for infrared radiation
(f)
E =4.0 X 10-2kJ/mol for microwave radiation.
(a) A compound with a strong absorptionat 1710 cm-1 contains a carbonyl group and is
either a ketone, an aldehyde, or an ester.
(b)
with a nitro, group has a strong absorption at 1540 cm-1.
~ A compound
.
(c) A compound showing both carbonyl (1720 cm-1) and -OH (2500-3000 cm-1 broad)
absorptions is a carboxylic acid.
I
~
Q
To use IR spec~oscopy to distinguish between isomers, find a strong IR absorption that is
present in one isomer but absent in the other.
.
(a)
CHsCH20H
CHSOCHS
Strong hydroxyl band
at 3400 - 3640 cm-1
(b)
.
CHsCH2CH2CH2CH
=CH2
Alkene bands at
3020-3100 cm-1 and
at 1640-1680-1.
.No band in the region .
3400 - 3640 cm-1
o
No bands in alkene region.
(c)
CHsCH2C02H
Strong, broad band
at 2500-3100 cm-1
HOCH2CH2CHO
Strong band at
.3400-3640 cm-1
@ 20 II Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a PUblicly accessible website, in whole or in part.
Structure
Determination:
Mass Spectrometry,
JR, and UV Spectroscopy
223
~ ~ (a) Anat 1640-1680
ester next to a double bond absorbs at 1715cm-I. The alkene double bond absorbs
D
cm-I.
(b) The aldehyde carbonyl group absorbs at 1730cm-I. The alkyne CaC bond absorbs at
2100-2260 cm-I, and the alkyne H-G bond absorbs at 3300 cm-I.
(c) The most importantabsorptions for this compound are due to the alcoholgroup (a
broad, intense band at 3400-3650 cm-I) and to the carboxylic acid group, which has a
C=O absorption in the range 1710-1760 cm-I and a broad O-H absorptionin the
range 2500-3100 cm-I. Absorptions due to the aromatic ring [3030 cm-I (w) and
1450-1600 cm-I (m)] may also be seen.
EJ
0.10
0
.
II
HsC
,
~
C
'CHs
H
The compound contains nitrile and ketone groups, as well as a carbon-carbon double
bond. The nitrile absorption occurs at 2210-2260 cm-I. The ketone shows an absorption
at 1690cm-I, a value lower than the usual value because the ketone is next to the double
bond. The double bond absorption occurs at 1640-1680 cm-I.
~
10.11
200 nm
= 200 x 1O-9m = 2 x 10-7m
400 nm
= 400 x 10-9m = 4 x 10-7 m
forA
E
= 2 x 10-7m:
= 1.20 X 10-4 kl/mol
=
1.20 X 10-4kl/mol = 6.0 x 102kl/mol
2.0 x 10-7
=
1.20 X 10-4 kl/mol
A (in ~)
for A = 4 x 10-7m:
E
= 1.20 X 10-4 kl/mol
4.0 x 10-7
A(in m)
= 3.0 x 102kl/mol
The energy of electromagneticradiationin the region of the spectrumfrom 200 om to 400
om is 300 - 600 kl/mol. Comparing this with the energy value for IR transitions
(calculatedin Section 10.6):
Energy (in kl/mol)
UV
IR
300 - 600
4.8 - 48
The energy required for UV transitions ~sgreater than the energy required for IR
transitions.
C 2011 Cengage I:earning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
L
224
Chapter
B..
10
A
Cxl
Where E = molar absorptivity(in L/mol.cm)
A '= absorbance
In this problem:
E = 50,100 = 5.01 x 104Llmol .cm
1 = 1.00 cm
A = 0.735
e
o .13
C
= -LL =
EX 1
1 = sample path length (in cm)
C
= concentration(inmol/L)
= 1.47 x 10-5M
0.735
5.01 X 104 Llmol.cm x 1.00 cm
11compounds having alternating single and multiple bonds should show ultraviolet
sorption in the range 200-400 nm. Only compound (a) is not UV-active. All of the
compoundspicturedbelowareUV active.
.
o
II
o
Visualizing
.
(JC
,
~
COH
OCCHs
II
o
Chemistry
10.14 (a) The mass spectrum of this ketone shows fragments resulting from both McLafferty
rearrangementand alpha cleavage.
McLaffertyrearrangement:
0
+.
CHsCH29H~CH2C?Hs
[
j
CHs
M+ 114
=
.
McLafferty
rearrangement
H ~ /H~
"9~
~
H_C~C"
H/
CH2CHs
IC\
H CHs
t
H'9
C
HsC" C~ "CH2CHs
I
H m/z = 86
II:)2011 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website: in whole or in part.
228
.
Chapter
10
=88. The fragment
(b) CSHIZO is the formula Qf an alcohol with M+
at m/z
=70 is due to
the product of dehydration ofM+. The other two fragments are a result of alpha cleavage.
Draw the possible Cs alcohol isomers, and draw their products of alpha cleavage. The
tert~aryalcohol shown fits the data.
+"
OH
+ b-+
CH3
I
CH3CH2
[
I
I
CH3
M+ =88
]
Alpha.
cleavage
+"
H3C,
/.CH3
C=C
.,
/
H
[
CH3]
m/z
CH3
I
=70
.
CH3CH2CH2CHCH3
2-Methylpentane
k
+"
CH
+e
[CH3CH2CH2bH~H3 ]
!
+
CH3CH2CH2CHCH3
m/z
=71
+
CH3
\
I
CH2CHCH3
m/z
=57
CH3
+1
CHCH3
m/z =43
The molecular ion, at m/z =86, is present in very low abundance. The base peak, at m/z =
43, represents a stable secondary carbocation.
.
Q
V
Before.doing the hydrogenation,familiarize yourself with the mass spectra of cyclohexene
and cyclohexane.Note that M+is different for each compound. After the reaction is
underway, inject a sample from the reaction mixture into the mass spectrometer.If the
reaction is finished, the mass spectrum of the reaction mixture should be superimposable
with the mass spectrum of cyclohexane.
iO 2011 Cengage Leaming. All Rights Reserved. May nol be scanned, copied or duplicated,
or poSled to a publicly accessible website, in whol~ or in part.
Structure
6
Determination:
Mass Spectrometry,
IR, and UV Spectroscopy
229
~5 (a) This ketone shows mass spectrumfragments that are due to alpha cleavage and to the
McLafferty rearrangement.The molecular ion occurs at W = 148,and major fragments
have m/z = 120, 105, and 71. (Note that only charged species are shown.)
°II
+"
+
Alpha ..
(tc
I
\
C1OH120
cleavage
I
M+
O
= 148
+
aO
mlz
= 105
m/z
+"
HYJ
II
C
McLafferty
..
'0
th
=71
H,
-, +"
°I
H2C
c'O
c'O
reammgement
r
l O."'C
m/z
I
= 120
(b) The fragments in the mass spectrum of this alcohol (CgH160)result from dehydration
and alpha cleavage. Major fragments have m/z values of 128 (the same value as the
molecular ion), 110 and 99.
M+ = 128
m/z
-M+= 128
mlz
=110
=99
m/z
= 128
(c) Amines fragment by alpha cleavage. In this problem, cleavage occurs in the ring,
producing a fragment with the same value of m/z as the molecular ion (99).
H
I
~N,
u--
M+=99
H
I
- Alpha
CHs
..
cleavage
~N'CH3
m/z =99
C>2011 Cengage Leaming_ All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
230
-c-
Chapter 10
(lO.26)CH3CH2CaCH
shows absorptions at 2100-2260 cm-I (CaC) and at 3300 cm-I (CaC-H)
"-J
that are due to the terminal alkyne bond.
H2C=CHCH=CH2 has absorptions in the regions 1640-1680 cm-I and 3020-3100 that
are due to the double bonds. It also shows absorptions at 910 cm-I and 990 cm-I that are
due to monosubstituted alkene bonds. No absorptions occur in the alkyne region.
CH3CaCCH3.For reasons we won't discuss, symmetrically substituted alkynes such as
but-2-yne do not show a CaC bond absorptionin the IR. There is also no terminal alkyne
absorption.This alkyne is distinguished from the other isomers in that it shows no
absorptions in either the alkyne or aUceneregions.
Two enantiomershave.identicalphysical properties (other than the sign of specific
rotation). Thus, their IR spectra are also identical.
Diastereomershave different physical properties and chemical behavior, and their IR
spectraare alsodifferent.
~
U
.
(a) Absorptions at 3300 cm-I and 2150 cm-I are due to a terminal triple bond. Possible
structures:
CH3CH2C~2C
= CH
(CH3)2CHC
= CH
(b)An IR absorptionat 3400cm-I is due to a hydroxylgroup.Sinceno doublebond
abs?rptionis present,the compoundmustbe a cyclicalcohol.
CH3
[>(
~OH
OH
J>-CH3
HO
(c) An absorption at 1715 cm-I is due to a ketone. The only possible structure is
CH3CH2COCH3.
(d) Absorptions at 1600 cm-I and 1500cm-I are du~ to an aromatic ring. Possible
structures:
CH3
~3\
~
(a)HCaCCH2NH2
Alkyne absorptions at
3300 cm-I, 2100-2260 cm-I
Amine absorptionat
3300-3500 cm~I
(b) CH3COCH3
Strong ketone absorption
at 1715 cm-I
CH3CH2CaN
Nitrile absorptionat
2210-2260 cm-I
CH3CH2CHO
Strong
absorption
at 1730aldehlde
cm-
C 20 II Cengage Leaming. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website. in whole or in part.
Structure Determination: Mass Spectrometry, IR, and UV Spectroscopy
~
&my
231
Spectrum (b) differs from spectrum (a) in several respects. Note in particularthe
absorptions at 715 cm-1 (strong), 1140 cm-1 (strong), 1650 cm-I (medium), and 3000
cm-I (medium) in spectrum (b). The absorptions at 1650 cm-I (C=C stretch) and 3000
cm-I (=C-H stretch) can be found in Table 10.1.They al10w.usto assign spectrum (b) to
cyc10hexeneand spectrum (a) to cyc1ohexane.
(a).
absorptionswithmediumto strougintensityare listed.
. C=c
(b)
o
~C/
OH
6
7"'
I
~
(c)
N
III
C
OH
(e)
aromatIcnng 1450-1600 cm-I
aromatic ring C-H
3030 cm-I
0
1710-1760 cm-I
carboxylicacid O-H
2500-3100 cm-I
monosubstituted
aromaticring
690-710 cm-I
730-770 cm-I
ester
1735 cm-I
OCH aromaticring C=C
1450-1600 cm-I
3
aromatic ring C-H
3030 cm-I
aromaticester
I
1715 cm-I
monosubstituted
aromaticring
690-710 cm-I
730-770 cm-1
6
7"'
carboxylicacidC=O
aromatic ring C=C
1450-1600 cm-I
aromatic ring C-H
3030 cm-I
alcoholO-H
3400-3650 cm-I
nitrile C=N
2210-2260 cm-I
p-disubstituted
aromaticring
810-840 cm-I
~C/
~
(d)
0
alkene C=C
1640-1680 cm-I
alkene =C.",H
3020-3100 cm-I
ketone
1715 cm-I
ketone
1715 cm-I
(b) CH3COCH=CHCH3,a ketone conjugated with a double bond, shows a strong ketone
absorption at 1690 cm-I; CH3COCH2CH=CH2shows a ketone absorption at 1715
cm-I and monosubstituted alkene absorp~ionsat 910 cm-I and 990 cm-I.
(c) CH3CH2CHOexhibits an aldehyde band at 1730 cm-I; H2C=CHOCH3shows
characteristic monosubstituted alkene absorptions at 910 cm-I and 990 cm-I.
Q 20 II Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part.
Structure Determination: Mass Spectrometry, IR, and UV Spectroscopy
235
10.43
H
CH3
I
I
'iC,
'iC,
'iCH2
H2C
C
C
I
I
H
CH3
Hexa-l,3,5-triene
Amax
2,3- Dimethylhexa-l,3 ,5-triene
Amax "'" 268 nm
= 258nm
In Problem10:42,weconcludedthatone alkylgroupincreasesAmaxof a conjugateddiene
by approximately 5 nm. Since 2,3-~imethylhexa-l,3,5-triene has two niethyl substituents,
its UV Amaxshould be about
10 nm longer than the Amaxof hexa-l ,3,5-triene.
,
10.44
C
~
=~
Exl
=
0.065
11,900L/mol'cmx 1.00cm
=
6.5 x 10-2
=' 5.5 x lO-6M
1.19x 104L/mol
;i 0.45 )The peak of maximum intensity (base peak) in the mass spectrum occurs at mlz =67. This
~~akdoes not represent the molecular ion, however, because M+of a hydrocarbonmust be
an even number. Careful inspection reveals the molecular ion peak at mlz =68. M+ =68
corresponds to a hydrocarbon of molecular formula CsHgwith a degree of unsaturationof
two.
Fairly intense peaks in the mass spectrum occur at mlz =67, 53, 40,39, and 27. The
~~
peak at mlz
=67 corresponds
to loss of one hydrogen atom, and the peak at mlz
=53
represents loss of "amethyl group. The unknown hydrocarbon thus contains a methyl
group.
Significant IR absorptions occur at 2130 cm-1 (-C=C- stretch) and at 3320 cm-1
(=C-H stretch). These bands indicate that the unknown hydrocarbon is a terminal alkyne.
Possible structures for CsHg are CH3CH2CH2C=CHand (CH3hCHC=CH. [Pent-l-yne
is correct.]
~
Ii 0.46 he molecular ion, M+ =70, corresponds to the molecular formula CsH10.This
\~compound
has one double bond or ring.
'
The base peak in the mass spectrum occurs at mlz
= 55. This peak represents
loss of a
methyl group from the molecularion and indicatesthe presence of a methyl group in the
unknown hydrocarbon. All otber peaks occur with low intensity:
In the IR spectrum, it is possible to distinguish absorptions at 1660 cm-1 and at 3000
cm-1 due to a double bond. (The 2960 cm-1 absorption is rather hard to detect because it
occurs as a shoulder on the alkane C-H stretch at 2850-2960 cm-1.)
Since no absorptions occur in the region 890 cm-1- 990 cm-1, we can exclude
terminal alkenes as possible structures.The remaining possibilities for CsH10are
CH3CH2CH=CHCH3and (CH3hC=CHCH3. [2-Methylbut-2-ene is correct.]
Q 2011 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a pllblicly accessible website, in whole or in part.