Engineering M12 Solutions Chapter 03
advertisement
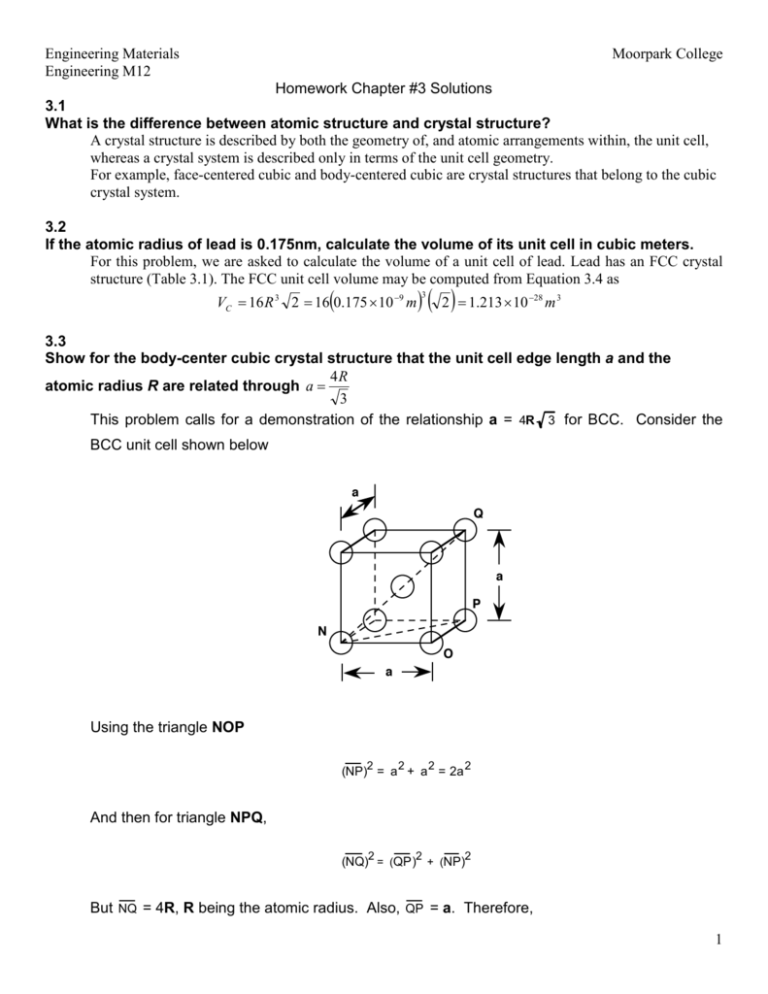
Engineering Materials Engineering M12 Moorpark College Homework Chapter #3 Solutions 3.1 What is the difference between atomic structure and crystal structure? A crystal structure is described by both the geometry of, and atomic arrangements within, the unit cell, whereas a crystal system is described only in terms of the unit cell geometry. For example, face-centered cubic and body-centered cubic are crystal structures that belong to the cubic crystal system. 3.2 If the atomic radius of lead is 0.175nm, calculate the volume of its unit cell in cubic meters. For this problem, we are asked to calculate the volume of a unit cell of lead. Lead has an FCC crystal structure (Table 3.1). The FCC unit cell volume may be computed from Equation 3.4 as ( VC = 16 R 3 2 = 16 0.175 × 10 −9 m ) ( 2 ) = 1.213 × 10 3 −28 m3 3.3 Show for the body-center cubic crystal structure that the unit cell edge length a and the 4R atomic radius R are related through a = 3 This problem calls for a demonstration of the relationship a = 4R 3 for BCC. Consider the BCC unit cell shown below Using the triangle NOP 2 2 2 (NP) = a + a = 2a 2 And then for triangle NPQ, 2 2 2 (NQ) = (QP) + (NP) But NQ = 4R, R being the atomic radius. Also, QP = a. Therefore, 1 Engineering Materials Engineering M12 Moorpark College 2 (4R) = a 2 a = 2 + 2a , or 4R 3 3.7 Molybdenum has a BCC crystal structure, an atomic radius of .1363 nm, and a atomic weight of 95.94 g/mol. Compute and compare it theoretical density with the experimental value found inside the front cover This problem calls for a computation of the density of molybdenum. According to Equation (3.5) ρ= nAMo VC A For BCC, n = 2 atoms/unit cell, and 4R 3 VC = 3 Thus, ρ= (2 atoms/unit cell)(95.94 g/mol) 3 3 -7 23 (4 ) 0.1363 x 10 cm / 3 / (unit cell) 6.023 x 10 atoms/mol ( ( ) ) = 10.21 g/cm3 3 The value given inside the front cover is 10.22 g/cm . 2 Engineering Materials Moorpark College Engineering M12 3.13 Niobium has a atomic radius of 0.1430 nm and a density of 8.57 g/cm3. Determine whether it has an FCC or BCC crystal structure. In order to determine whether Nb has an FCC or BCC crystal structure, we need to compute its density for each of the crystal structures. For FCC, n = 4, and a = 2 R 2 . Also, from Figure 2.6, its atomic weight is 92.91 g/mol. Thus, for FCC ρ = nANb 3 2R 2 N A ( ) (4 atoms/unit cell)(92.91 g/mol) 3 -8 2 /(unitcell) 6.023 x 10 23 atoms / mol (2) 1.43 x 10 cm = ( )( ) ( ) = 9.33 g/cm3 For BCC, n = 2, and a = = 4R , thus 3 (2 atoms/unit cell)(92.91 g/mol) 3 -8 (4) 1.43 x 10 cm 23 /(unitcell) 6.023 x 10 atoms/ mol 3 ( ) ( ) = 8.57 g/cm3 which is the value provided in the problem statement. Therefore, Nb has a BCC crystal structure. 3 Engineering Materials Moorpark College Engineering M12 3.17 Beryllium has a HCP unit cell for which the ratio of the lattice parameters c/a is 1.568. If the radius of the Be atom is 0.1143 nm. Determine the unit cell volume. For HCP, there are the equivalent of six spheres per unit cell, and thus 4π R3 3 VS = 6 = 8πR 3 Now, the unit cell volume is just the product of the base area times the cell height, c. This base area is just three times the area of the parallelepiped ACDE shown below. The area of ACDE is just the length of CD times the height BC . But CD is just a or 2R, and BC = 2R cos (30°) = 2R 3 2 Thus, the base area is just 2R 3 2 AREA = (3)(CD)(BC) = (3)(2 R) = 6R 3 2 VC = 6R2c 3 But, c = 1.568a, and a = 2R, or c = 3.14R, and 4 Engineering Materials Engineering M12 Moorpark College VC = (6)(3.14) R3 3 ( )[0.1143 x 10-7 cm] 3 = (6) (3.14 ) 3 = 4.87 x 10 −23 3 cm /unit cell Calculate the theoretical density of Be and compare it with the literature value. ρ = nA Be VC NA For HCP, n = 6 atoms/unit cell, and for Be, A = 9.01 g/mol. Thus, Be ρ = (6 atoms/unit cell)(9.01 g/mol) -23 3 23 4.87 x 10 cm /unit cell 6.023 x 10 atoms/mol ( )( ) = 1.84 g/cm3 3 The value given in the literature is 1.85 g/cm . 5