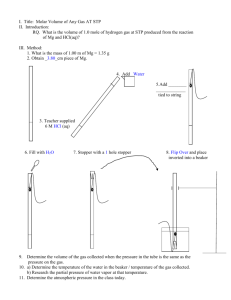
Calculating Gas Density At Depth
by
Larry "Harris" Taylor, Ph.D.
This material is copyrighted and all rights retained by the author. This article is made
available as a service to the diving community by the author and the Occupational Safety
and Environmental Health Department of the University of Michigan. It may be distributed
for any non-commercial or Not-For-Profit use
All rights reserved.
This article assumes a basic understanding of the common gas law properties.
See A Gas Law Primer for review of these concepts.
Calculating Gas Density At STP
The change in density as a result of change in chemical composition of a gas mix can be easily
determined using well-established chemical principles. It is fact that 1 mole (the molecular mass
of a substance expressed in grams) of any gas occupies 22.4 liters at STP (standard temperature
and pressure: 0 oC (273 K); 1 ata). Tables of molecular weights can be found in any elementary
chemical text. These tables tell us that:
molecular mass of O2: 31.998 amu (atomic mass units)
molecular mass of N2: 28.014 amu
molecular mass of He: 4.00 amu
Density is defined as mass / volume. Since one mole of dry gas at STP occupies 22.4 liters, the
density of a pure substance is easily determined:
Density =
Mass
Volume
Density O2 = 31.998 g/mole x 1 mole/22.4 L = 1.428 g/L
Density N2 = 28.014 g/mole x 1 mole/22.4 L = 1.251 g/L
Density He = 4.00 g/mole x 1 mole/22.4 L = 0.178 g/L
Oxygen enriched air (EANx or Nitrox) is a binary mixture of nitrogen and oxygen. Thus, the mass
for the mix can be determined by simply summing the masses of the individual components. For
example, by choosing a volume of 1 liter, the density at STP, of 32 % oxygen containing mix
(NOAA 1) can be calculated.
Mass = Density x Volume
For NOAA I (32 % O2 )
For NOAA II (36 % O2 )
Oxygen mass in 1 liter of mix: 0.32 (1.428 g/L) (1 L) = 0.4569 g
Nitrogen mass in 1 liter of mix:: 0.68 (1.251 g/L) (1 L) = 0.8507 g
______________________________________________________
Mass of NOAA I mix occupying 1 liter at STP:
1.3076 g
Oxygen mass in 1 liter of mix: 0.36 (1.428 g/L) (1 L) = 0.5141 g
Nitrogen mass in 1 liter of mix: 0.64 (1.251 g/L) (1 L) = 0.8507 g
_____________________________________________________
Mass of NOAA II mix occupying 1 liter at STP:
1.3648 g
This method, as long as components are known, can be applied to any mixture of gases. For
example, the density of Tri-mix 21/50 calculates to be 0.75196 g/L. This can be compared to the
value of dry air at STP listed in the CRC HANDBOOK OF CHEMISTRY AND PHYSICS of 1.296
g/L.
Density of Dry Gases At STP
(g/L)
Air:
2960
NOAA I
1.3076
NOAA II
1.3648
Tri-mix 21/50
0.75196
Calculating Gas Density at Depth
Since the pressure changes associated with scuba diving at recreational depths are relatively
small, we may assume ideal gas behavior. With this assumption, the gases will behave according
to Boyle's law and density will be directly proportional to absolute pressure. For the direct
comparison of air with oxygen enriched air, let's examine a "worst case" scenario: diver breathing
dry gas at 0 oC at 132 FSW. (Note that 132 FSW exceeds the recommended depth for NOAA II;
also 0 oC is much colder than waters where divers normally play.) These values was chosen, as
an illustration, to maximize the density differences observed.
First. determine the absolute pressure at 132 fsw:
Absolute Pressure = Water Column Pressure + Atmospheric Pressure
water pressure = 132 ft / 33 ft/atm = 4 atm
absolute pressure = 4 atm + 1 Atm = 5 ata
Since density is directly proportional to absolute pressure:
Density of Dry Gases At 132 fsw
Air:
NOAA I :
NOAA II :
Tri-mix 21/50
1.296 g/L x 5 = 6.48 g/L
1.308 g/L x 5 = 6.54 g/L
1.315 g/L x 5 = 6.58 g/L
0.752 g/L x 5 = 3.76 g/L
2
Conclusion
Assuming ideal gas behavior allows basic chemical principles to be used to estimate gas density
of a dry gas at recreational depths. It should be noted that mixes with helium often do not display
ideal gas behavior. Also, as depth increases well beyond the recreational limit, gas behavior
departs from predictions of ideal relationships and more complex real gas equations must be
used. Although this simple method offers a reasonable estimate of gas densities, it should not be
considered "gospel" for all mixes at all depths.
About The Author:
Larry "Harris" Taylor, Ph.D. is a biochemist and Diving Safety Coordinator at the University of
Michigan. He has authored more than 100 scuba related articles. His personal dive library (See
Alert Diver, Mar/Apr, 1997, p. 54) is considered one of the best recreational sources of
information In North America.
Copyright 2004 by Larry "Harris" Taylor
All rights reserved.
Use of these articles for personal or organizational profit is specifically denied.
These articles may be used for not-for-profit diving education
3