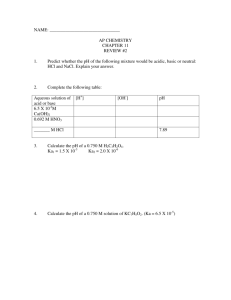
1) Answer the following questions about laboratory

!
!
Ch. 4 Acid-Base APQs !
Name:
Period:
1) Answer the following questions about laboratory situations involving acids and bases.
(a) Propanoic acid, HC
3
H
5
O
2
, reacts with water to produce an acidic solution. Shown below are the complete Lewis structures of the reactants.
+ !
+
In the space provided above, complete the equation by drawing the complete Lewis structures of the reaction products.
(b) Choosing from the chemicals and equipment listed below, describe how to prepare
100.00 mL of a 1.00 M aqueous solution of NH
4
Cl (molar mass 53.5 g mol -1 ). Include specific amounts and equipment where appropriate.
NH
4
Cl
(s)
!
50 mL buret !
100 mL graduated cylinder !
100 mL pipet
Distilled water !
100 mL beaker !
100 mL volumetric flask
1- Weight out 5.35g of NH
4
Cl with the balance
!
Balance
2- Fill the 100mL volumetric flask with about 25-50mL of water using graduated cylinder
3- Add the 5.35g of NH
4
Cl
4- Fill to the mark, for a total volume of 100.00 mL of solution
(c) 25.0mL of 0.150M propanoic acid is added to 35.0mL of 0.100M potassium hydroxide. What would be the concentration of the excess reactant? (assume that the volumes are additive)
HC
3
H
5
O
2
+ KOH --> KC
3
H
5
O
2
+ H
2
O
HC
3
H
5
O
2
: 0.025L•0.150M= 0.00375 mol HC
3
H
5
O
2
KOH: 0.035L•0.100M= 0.00350 mol KOH
Based on a 1:1 ratio we will have 0.00025mol HC
3
H
5
O
2
left over, the total volume of the new solution would be 60.0mL or 0.0600L giving a concentration of:
0.00025mol / 0.0600L= 0.00417M HC
3
H
5
O
2
left in the solution.
2)For each of the following three reactions, in part (i) write a balanced equation for the reaction and in part (ii) answer the question about the reaction. In part (i), coefficients should be in terms of lowest whole numbers. Assume that solutions are aqueous unless otherwise indicated. Represent substances in solutions as ions if the substances are extensively ionized. Omit formulas for any ions or molecules that are unchanged by the reaction.
(a)
(i) Solid strontium hydroxide is added to a solution of nitric acid.
Sr(OH)
2(s)
+ 2H +
(aq)
Sr 2+
(aq)
+ 2H
2
O
(l)
(ii) How many moles of strontium hydroxide would react completely with 500. mL of 0.40 M nitric acid?
.500L•.40M=.20mol HNO
3
.20mol HNO
3
/2 = 0.10mol Sr(OH)
2
(b)
(i) Excess nitric acid is added to solid calcium carbonate.
2H +
(aq)
+ CaCO
3(s)
Ca 2+
(aq)
+ CO
2(g)
+H
2
O
(l)
(ii) Briefly explain why statues made of marble (calcium carbonate) displayed outdoors in urban areas are deteriorating.
Air pollution, mostly nitrogen and sulphur compounds, reacts with water in the air and creates acid rains. The acidic rain dissolves the calcium carbonate.
(c)
(i) Equal volumes of equimolar solutions of ammonia and hydrochloric acid are combined.
NH
3(aq)
+ H +
(aq)
NH
4
+
(aq)
(ii) Indicate whether the resulting solution is acidic, basic, or neutral. Explain.
The solution would be acidic, the NH
4
+ functions as an acid. (Don’t worry about this, we’ll talk about it later)