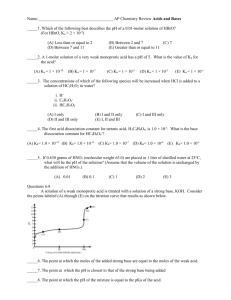
AP Chemistry Acid-Base Review Questions
advertisement
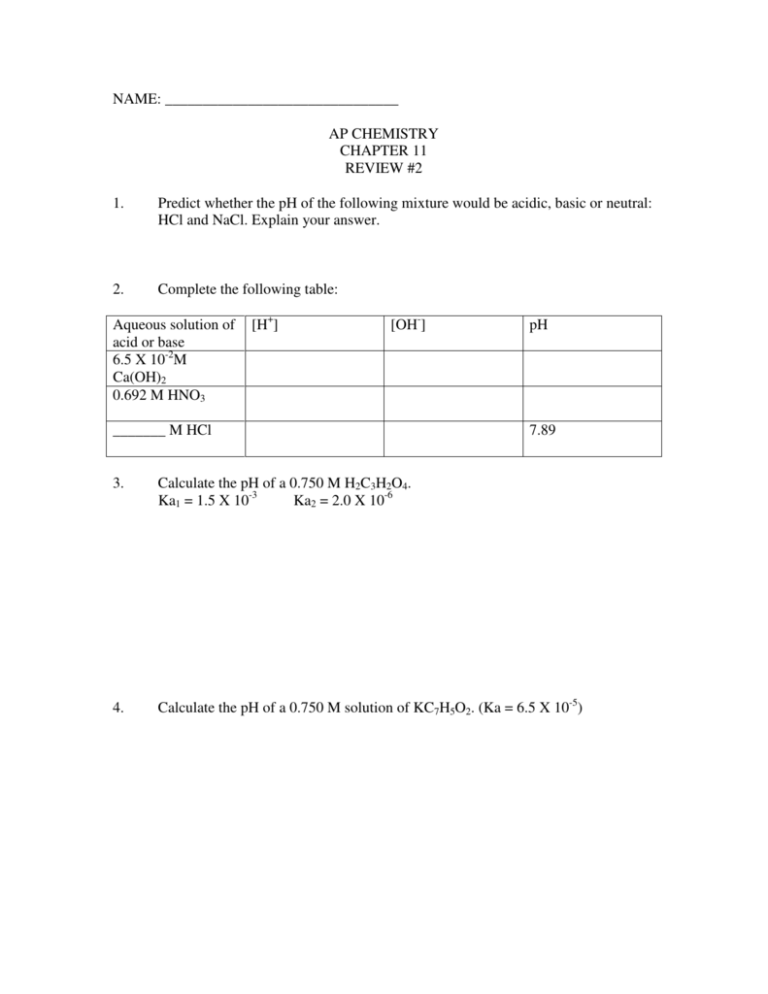
NAME: _______________________________ AP CHEMISTRY CHAPTER 11 REVIEW #2 1. Predict whether the pH of the following mixture would be acidic, basic or neutral: HCl and NaCl. Explain your answer. 2. Complete the following table: Aqueous solution of acid or base 6.5 X 10-2M Ca(OH)2 0.692 M HNO3 [H+] [OH-] _______ M HCl pH 7.89 3. Calculate the pH of a 0.750 M H2C3H2O4. Ka1 = 1.5 X 10-3 Ka2 = 2.0 X 10-6 4. Calculate the pH of a 0.750 M solution of KC7H5O2. (Ka = 6.5 X 10-5) 5. Calculate the pH and equilibrium concentrations of all species present in a 0.250 M HClO solution. 6. Calculate the pH of a solution made by mixing 50.0 mL of a 0.450 M Ca(OH)2 solution with 25.0 mL of a 0.639 M solution of NaOH. 7. Calculate the equilibrium constant for an aqueous solution of a weak base if the solution for the weak base is 0.750 M and has a pH of 10.15. 8. A solution of calcium hydroxide is titrated with a solution of HOCl. a. Calculate the volume of a 0.215 M calcium hydroxide needed to reach the equivalence point when titrated into a 75.0 mL sample of 0.136 M HOCl. 9. b. Indicate whether the pH at the equivalence point is less than 7, equal to 7 or greater than 7. You must explain your answer. c. HOCl is a weaker acid than HClO4. Explain why this statement is true. The acid ionization constant, Ka, for propanoic acid, C2H5COOH, is 1.3 X 10-5. a. Calculate the hydrogen ion concentration in a 0.20 M solution of propanoic acid. b. Calculate the percentage of propanoic acid molecules that are ionized in solution (a). c. Determine the pH for the solution in (a). 10. Calculate the pH of a 0.0500 M solution of H2SO4. 11. Calculate the pH of a 0.30 M solution of iodic acid (HIO3, Ka = 0.17). 12. Calculate the pH of a solution containing a mixture of 0.25 M HNO3 and 0.25 M HF. 13. Predict if the following solution would be acidic, basic or neutral? NaF 14. Place the following groups in order of increasing acid strength: a. NH4+, HONH3+ 15. 16. b. NH4+, PH4+ (bond energies: N-H = 391 kJ/mol; P-H = 322 kJ/mol) c. HClO2, HBrO2, HIO2 d. HBrO, HBrO3, HBrO2 Identify the Lewis acid and the Lewis base in each of the following: a. B(OH)3 + H2O B(OH)4- + H+ b. Ag+ c. BF3 + NH3 FBNH3 + 2NH3 - Ag(NH3)2+ The pH of a .016 M solution of p-toluidine (CH3C6H4NH2) in water is 8.60. Calculate the Kb.