Identifying a Liquid Using Physical Properties1

Identifying a Liquid Using Physical Properties
1
Thomas M. Moffett Jr., SUNY Plattsburgh, 2009
Objectives
* Determine the density of an unknown liquid by measuring mass and volume.
* Determine the solubility of an unknown liquid in water and cyclohexane.
* Determine the boiling point of an unknown liquid.
* Use the above physical properties to determine the identity of an unknown liquid.
Introduction
Physical properties are properties that can be measured without changing the identity of the sample. These properties can fall into one of two categories. Properties that are dependent on the size of the sample are said to be extensive properties. Examples of extensive properties include mass, volume, and heat. Properties that depend solely on the material and not the size of the sample are intensive properties. Intensive properties can be used to help identify chemicals. In this experiment you will measure three intensive properties, density, boiling point, and solubility, in order to determine the identity of an unknown volatile liquid. Volatile liquids are liquids which evaporate easily.
Density
Density is the ratio of an object’s mass to its volume. d = m v
(1)
As the equation above shows, the intensive property of density is actually the ratio of two extensive properties. In fact the ratio of any two (related) extensive properties will always result in an intensive property, since both properties will increase proportionally with the size of the sample.
Solubility
The solubility of a solute in a solvent depends on several factors, these include temperature and intermolecular interactions. Intermolecular interactions are the attractive forces that exist between molecules. In order for a solute to dissolve in a solvent the interactions between solute and solvent molecules must be of approximately equal strength as the interactions between
! " ! #
$ % & # ''(
solvent molecules and the interactions between solute molecules. Another way of stating this is
“Like dissolves like”. This rule refers to the fact that polar molecules will dissolve in other polar molecules and non-polar molecules will dissolve in non-polar molecules. An example that you are familiar with is oil and vinegar salad dressing, no matter how much you shake up the dressing it will always separate into two layers. In this case the oil is non-polar and the vinegar is polar.
Polar molecules have regions of permanent charges, from an unequal distribution of electrons.
While the electrons in a non-polar molecule are evenly distributed throughout the molecule, resulting in no separation of charge centers.
Figure 1: Electrostatic potential surfaces of water (left) and cyclohexane (right). The red areas are negatively charged and the blue areas are positively charged. Notice that in the water molecule (polar) the charges are not evenly distributed, while in cyclohexane (non-polar) the charges are evenly spread out around the molecule.
If two chemicals are able to mix together and form a solution they are said to be miscible, or soluble in each other. If the solute does not dissolve into the solvent it is insoluble, when this occurs the two liquids will form separate layers. Some solutes may also be classified as slightly soluble, where one or two drops will dissolve, but additional drops will not go into solution.
Boiling Point
Boiling is the physical change of state from a liquid to a gas. In order for a liquid to become a gas it must overcome the intermolecular attractions holding the molecules together in the liquid.
To overcome these forces energy needs to be applied in the form of heat (thermal energy). As the molecules absorb heat, their kinetic energy increases, eventually to the point where the molecules can escape from the liquid sample. Kinetic energy is the energy of motion, recall that temperature is a measure of average kinetic energy. The stronger the interactions are between liquid molecules, the more energy the molecules require to evaporate. Thus the stronger the interactions, the higher the boiling point will be. In general polar molecules have higher boiling points than non-polar molecules of comparable size, and larger molecules have higher boiling points than smaller molecules.
When a liquid boils its temperature will remain constant (even as more heat is applied). This temperature is referred to as the boiling point. The boiling point is dependent upon the external pressure, as pressure increases so does boiling point. The temperature a liquid boils at when pressure equals 1 atm is referred to as the normal boiling point. A pressure of 1 atm is the average atmospheric pressure at sea level. As you gain in elevation, pressure (and boiling point) decreases.
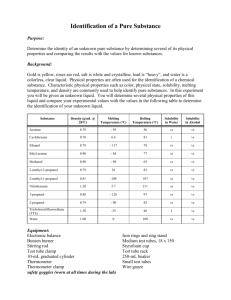
Compound
Density
(g/mL)
Normal Boiling
Point ( o C)
Solubility in
Water
Solubility in cyclohexane acetone methyl acetate
1-hexene methanol hexane methyl cyclopentane ethyl acetate ethanol
Methyl propionate cyclohexane
2-proponol isopropyl acetate ethyl propionate
1-propanol water 1.00
Table 1 - Physical properties of some liquids
0.79
0.92
0.78
0.78
0.87
0.89
0.80
0.79
0.93
0.67
0.79
0.66
0.75
0.90
Procedure 2
78.5
80
81
82
85
99
100
100
56
57.5
63
65
69
72
77 s s s i s s s s s s i i i s sl. s
A.
Density
1.) Obtain about 15 mL of the assigned unknown liquid in a medium size test tube.
2.) Rinse a 5 mL volumetric pipette with a small amount of the unknown.
3.) Use the pipette to transfer 5 mL of the unknown liquid into a clean dry pre-weighed beaker, record the mass of the beaker.
4.) Return the unknown sample to the test tube.
5.) Repeat steps 2 – 4 with a 10 mL volumetric pipette s s i sl. s s s s s s s s sl. s s s s
)
$ % & # ''(
! " ! #
B.
Solubility
1.) Add approximately 1 mL of DI water to a small test tube.
2.) Add one or two drops of your unknown liquid to the test tube. Cap the test tube and shake vigorously. If the unknown appears to be soluble, add three to five more drops to the test tube, shake and observe.
3.) Dispose of the test tube contents in the appropriate waste container.
4.) Repeat steps 1 - 3 using cyclohexane in place of DI water.
C.
Boiling Point
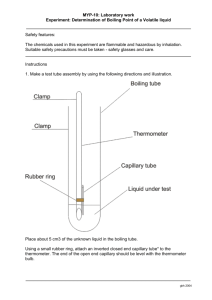
1.) Set up a boiling point apparatus as shown in Figure 2. The thermometer may be attached to the test tube with a rubber band rather than using a second clamp. The bottom of the test tube and thermometer should be at the same level, approximately 2 cm above the bottom of the beaker. It is a good idea to put a second iron ring around the beaker to keep it from tipping over.
2.) Add approximately 1 mL of the unknown liquid to the test tube. Add a capillary tube that is sealed at one end to the test tube. The open end must be down. If the capillary tubes have two open ends your instructor will show you how to seal one end.
3.) Add water and one or two boiling stones to the beaker. The water level should be above the level of the unknown liquid in the test tube.
4.) Use the Bunsen burner to slowly heat the water bath. While heating use a glass stirring rod to stir the water.
5.) Continue heating until you see a string of bubbles emerging from the capillary tube. At this point, turn off the Bunsen burner. Record the temperature when the bubbles stop coming out of the capillary tube, this is the boiling point of the unknown liquid.
6.) Carefully transfer the unknown liquid to the appropriate waste container. The used capillary tube should be placed in the broken glassware box.
7.) Add another 1 mL of your unknown liquid to your test tube and a new capillary tube. Repeat the boiling point procedure.
Figure 2: Boiling point apparatus
Sample Data Sheets
Unknown: _______________
A. Density Data
Mass of beaker and unknown liquid _______________ _______________
Mass of beaker
Mass of unknown liquid
Volume
Density
Average Density
_______________
_______________
_______________
_______________
_______________
_______________
_______________
_______________
_____________
B Solubility
Solvent
Water
Cyclohexane
Appearance after shaking soluble (s / I / sl. s.)
C. Boiling Point
Boiling point of unknown liquid _______________ _______________
Average boiling point
Identity of the unknown
Density %-error
Boiling Point %-error
______________
______________
______________
______________
Post Lab Questions
Q1) Was your unknown liquid polar or non-polar, explain your answer.
Q2) Would the boiling point of your unknown liquid be too high or too low if the thermometer bulb was touching the bottom of the beaker?
Q3) If your unknown liquid was insoluble in water, did it form a layer above or below the water? Explain your answer. Likewise if your unknown liquid was insoluble in cyclohexane, did it form a layer above or below the cyclohexane? Explain your answer. If your unknown liquid was soluble in both, write “soluble in both”.
Q4) Which of the measurements would be most affected if this experiment was repeated on top of Mt. Marcy (1629 m)?
Pre Lab Questions
P1) Define the following terms:
Normal boiling point –
Like dissolve like –
Intensive properties –
P2) Methane is a compound with the following properties. For each property determine whether it is a physical property or a chemical property.
a.) It is colorless gas at room temperature b.) Reacts explosively with oxygen. c.) Has a melting point of –182 o C d.) Has a molar mass of 16.04 g/mol
P3) Draw the NFPA diamonds for the following compounds (look at the MSDS sheets). a.) cyclohexane b.) ethanol