CHM 212 Experiment 4: Determination of the Ka of Potassium
advertisement

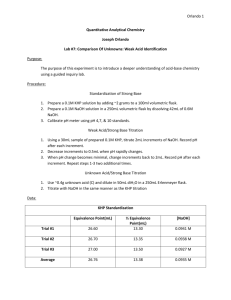
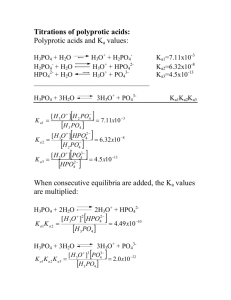
CHM 212 Experiment 4: Determination of the Ka of Potassium Hydrogen Phthalate (KHP) Using a Gran Plot Purpose: You will calculate the pKa of KHP by constructing a Gran plot from a titration curve. Your analysis will account for the effects of solution activity. Background See Section 10-5 of your textbook. In this experiment you will use a Gran plot to determine the acid dissociation constant (Ka) of an acid, potassium hydrogen phthalate (KHP). You will construct a titration curve of the KHP with sodium hydroxide (below) and then use a Gran plot and accounting for the effects of solution activity, you will calculate the Ka of the KHP. CO2K CO2K + NaOH + CO2H HA- H2O CO2Na A2- Note: When using the Gran plot, the generic acid “HA” is here “HA-“ and the generic base “A-“ is “A2-“. So where the Gran plot-related equations in the textbook call for a value relating to “HA”, use the corresponding value for “HA-“—I have made the change here already. It is critical that you collect sufficient data points in the region of the equivalence volume, Ve, because you will prepare the Gran plot (below) using data for which the volume of added base, Vb, is between 0.9Ve and Ve. Based on the accurately weighed amount of KHP and the accurately known concentration of standard NaOH, you may readily calculate Ve before beginning your titration. Additionally, you will account for the effects of solution activity in your calculation of Ka in this experiment. This involves calculating the ionic strength () of the solution and using this to determine the activity coefficients () of HA- and A2- (see the reaction above). Prelab Questions 1. Does it matter if you accurately weigh out the KHP that you add to the volumetric flask? 2. After you have rinsed your pH electrode after calibrating, what should you “wash” the pH electrode with before putting it in your analyte solution? Procedure: 1. Dry about 1.6 grams of the acid (potassium hydrogen phthalate – KHP) at 1100C for 1 hour and cool it to room temperature in a dessicator. 2. Accurately weigh (i.e. using the analytical balance) out ~ 1.5 grams of the acid and dissolve it in water in a 250 mL volumetric flask, using the stepwise procedure from earlier experiments. 3. Calibrate your pH meter using the supplied buffers. After calibration, rinse the electrode very well with distilled water and blot dry with kimwipes and then rinse in a small amount of your analyte solution. 4. Transfer 100mL of the acid solution to a 250mL beaker using a transfer pipet. Add a stir bar and position the setup in a way that the stir bar does not strike the pH electrode. Make sure that the pH meter is properly immersed in the solution. Allow the electrode to equilibrate approximately 1 minute with stirring and measure and record the initial pH of the solution. 5. Add 3 drops of phenolphthalein indicator and titrate the solution with standard ~ 0.1 M NaOH. It is VERY IMPORTANT THAT YOU DO NOT USE YOUR NaOH SOLUTION FROM EXPERIMENT 1. NaOH solution has been prepared and standardized for you. Be sure to record the exact molarity and the standard deviation for this solution as you will need to know these quantities later. 6. Add base in 1.5 mL increments recording the volume and the pH 30 s after each addition until you are within approximately 4 mL of the theoretical equivalence point calculated from the NaOH molarity and the amount of KHP. 7. When you are within approximately 4 mL of the equivalence volume, add the base in 0.4 mL increments, recording the volume and pH after each addition until you are within 1 mL of the equivalence point. After that, add base 1 drop at a time, again recording volume and pH after each addition until you have passed the pink endpoint by a few tenths of a mL. Add five more 1 mL aliquots beyond the pink endpoint. Record the exact volume at which the pink color is observed. 8. Construct a graph of pH vs Vb (volume of added base). Locate the equivalence volume (Veest) as the maximum point/slope from your first derivative plot or the point of zero second derivative from your second derivative plot as you did in experiment 2. Compare these volumes with the theoretical equivalence point and the phenolphthalein endpoints. 9. Put three sintered glass funnels in the oven at 100°C so they are dry for your next lab period. Calculations: 1. Construct a Gran plot (graph of Vb10-pH vs. Vb) by using the data collected in the region of 0.9 Veest and Veest. Label more clearly than the plot above—it should be possible to understand the plot at a glance (this should suggest to you that you should make sure to read the relevant section in the text). Draw a line through the linear portion of this curve and extrapolate it to the abscissa to find Ve. Use this value of Ve in the calculations below. Compare this value with those Veest found with phenolphthalein and the graphs of step 8 above. 2. Compute the slope of the Gran plot equation (Equation 10-5 in your text) to determine the Ka of KHP as follows: The slope of the Gran plot is . In this equation A2- is the phthalate anion (as indicated in the reaction above) while HA- is the monohydrogen phthalate ion. Because the ionic strength changes slightly as the titration proceeds, so also do the activity coefficients. Calculate the ionic strength at 0.95Ve and use this “average” ionic strength to find the activity coefficient (below). 3. You can consider the OH- and H+ concentrations to be negligible compared to the remaining ion concentrations, so the only ions whose concentrations you must consider in the calculation of ionic strength are [K+], [Na+], [HA-] and [A2-]. Recall that the ionic strength is calculated as (Equation 7-3 in your text) 4. In determining the activity coefficients of HA- and A2-, you can assume a hydrated radius of 6000pm for both of these ions. 5. From the measured slope of the Gran plot and the values of HA- and A2-, calculate the pKa. Then choose an experimental point near 1/3Ve and one near 2/3Ve. Calculate the pKa at each of these points using the Henderson Hasselbalch equation: In this equation, A2- is the ase and the HA- is the acid; use the Ka for the acidic form, HA-. 6. You will need to calculate [A2-], [HA-], HA- and A2- at each point. Calculate the average pKa values from your experiment and report that value along with its standard deviation. Below is an example of how ionic strength is calculated—check it over for errors and to make sure that you understand it! Example: find HA- and A2- at 0.95 Ve in the titration of 100.0 mL of 0.0200 M KHP with 0.100 M NaOH. Need to calculate the ionic strength, . [H+] and [OH-] are negligible relative to the other ion concentrations: [K+], [Na+], [HA-] and [A2-]. Ve = 20.0 mL, so 0.95 Ve = 19.0 mL. where is the initial solution volume, is the initial potassium ion concentration in that solution (equal to the initial concentration of KHP), is the new solution volume and is the new potassium ion concentration. where is the standardized sodium hydroxide concentration. where of HA- and is its original concentration before titration with NaOH. is the initial number of moles From this, the calculation of the ionic strength is straightforward. Using the given ionic radii of 6000pm for both HA- and A2- you can now determine the activity.