Titrations of polyprotic acids:
advertisement

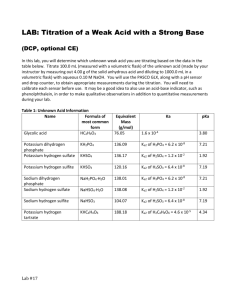
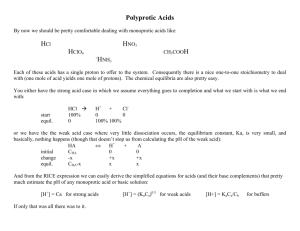
Titrations of polyprotic acids: Polyprotic acids and Ka values: H3PO4 + H2O H3O+ + H2PO4H2PO4- + H2O H3O+ + HPO42H3O+ + PO43HPO42- + H2O __________________________________ 3H3O+ + PO43- H3PO4 + 3H2O [ ] [ ] K a1 [ H 3 O + ] H 2 PO4− = = 7.11x10 −3 [H 3 PO4 ] Ka2 [ H 3O + ] HPO42 − −8 = = 6 . 32 x 10 H 2 PO4− [ [ ] Ka1=7.11x10-3 Ka2=6.32x10-8 Ka3=4.5x10-13 Ka1Ka2Ka3 ] [ H 3O + ] PO43− K a3 = = 4.5 x10−13 2− HPO4 [ ] When consecutive equilibria are added, the Ka values are multiplied: H3PO4 + 2H2O 2H3O+ + HPO42[ H 3O + ]2 HPO42− K a1 K a 2 = = 4.49 x10 −10 [H 3 PO4 ] [ H3PO4 + 3H2O K a1 K a 2 K a 3 ] [ 3H3O+ + PO43- ] [ H 3O + ]3 PO43− = = 2.0 x10 −22 [H 3 PO4 ] Titrations of polyprotic acids: multiple endpoints observable when Ka,n/Ka,n+1>103 Titration curve of a weak diprotic acid H2A: 1. pH before titration 2. pH before first equiv. point 3. pH at first equiv. pt. 4. pH between equiv. pts. 5. pH at second equiv. pt. 6. pH after second equiv. pt. H2A + H2O HA- + H2O H3O+ + HAH3O+ + A2- Ka1 Ka2 1. pH prior to titration: for a strong diprotic acid, same as strong acid for a weak diprotic acid, if Ka1 > 103 Ka2, second equilibrium makes little contribution assuming autoprotolysis contributes little + [ H ] ≅ K a1CH 2 A or 2. − K a1 + K a21 + 4 K a C H 2 A [H ] = 2 + pH prior to first equiv. pt., 1st buffer region 1st buffer region, both H2A and HA- present if Ka1 > 103 Ka2, second equilibrium makes little contribution, pH calculated like a normal buffer solution half way to equivalence, CH2A = CHA- [H+] = Ka1 3. pH at first equiv. pt. solution is like that of a salt of a diprotic acid (e.g., NaHA) [H ] = + [ [ ] ] K a 2 HA − + K w 1 + HA − K a1 If it can be assumed that [HA-] ≈ CNaHA [H ] ≈ + K a 2C NaHA + K w 1 + C NaHA K a1 [ ] + If CNaHAKa1 > 10-13 and CNaHA/Ka1 > 100, H ≈ K a1K a 2 4. pH in 2nd buffer region 2nd buffer region, both HA- and A2- present if Ka1 > 103 Ka2, first equilibrium makes little contribution, pH calculated like a normal buffer solution half way to equivalence, CHA- = CA2- [H+] = Ka2 5. pH at the second equivalence point Like a salt of A2-, main equilibrium is A2- + H2O OH- + HA- [ ][ Kw OH − HA− K b1 = = Ka2 A 2− [ ] ] [OH − ] ≅ K b1C A2 − or a more sophisticated relationship, if necessary 6. pH beyond 2nd equiv. pt. treated like the addition of strong base to water Two common types of titration curves are used to determine equivalence points for any kind of titration: sigmoidal curves and linear-segment curve: linear segment curve Depends upon difference in instrument response between reactants and products. Intersection of response lines before and after equivalence point determined location of equivalence point. Data typically collected far from equivalence point. E.g. titration leading to complex formation Analyte + Reagent → Complex lo response hi response lo response Analyte + lo response Reagent → lo response Complex hi response Sigmoidal curve Plot(s) of p-function of analyte (e.g., pH or pOH) versus reagent volume. Careful measurements made near the equivalence point. Acid-base titrations usually make use of this approach. Reagent solutions are almost always a standardized solution of a strong acid or strong base because they give sharper end points than do weak acids or bases. Identifying equivalence points: 1. titration with indicators (sigmoidal curve) 2. titration with linear-segment curve 3. titration monitored with a pH meter 4. Gran plot (see feature 14-5, text) Titration monitored with a pH meter: 1st derivative shows point of greatest slope – eq. pt. 2nd derivative indicates inflection point – eq. pt. A bit about indicators: Characteristics of analytically useful chemical reactions: 1. 2. 3. Reactants and products are easily distinguished The reaction provides useful information The reaction proceeds at high rates/efficiencies most acids and their conjugate bases are “transparent” to visible radiation pH indicators are exceptions, proton transfer reactions involving indicators meet the criteria for an analytically useful reaction pH transition range for an indicator: [H3O+] + [In-] HIn + H2O [H O ][In ] = + Ka − 3 [HIn] [ H 3O]+ = K a [HIn] [In ] − color changes at ratios and ≤ [HIn]/In-] = 0.1 ≥ [HIn]/[In-] = 10.0 cannot be discerned by eye Hence, useful range for a pH indicator is: pH = pKa±1 Note: concentration of indicator must be minimized to avoid introduction of systematic error