Lab: Feelin' the Heat
advertisement

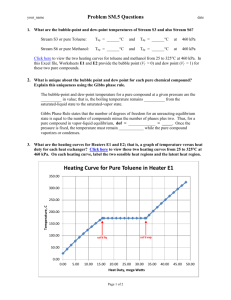
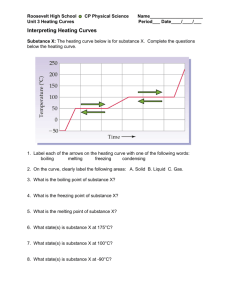
Name:_____________________________________ Period:__________ Date Due: _______________ ______/28 Points Lab: Feelin’ the Heat Question: How does the temperature change as a pure substance melts? 1. Imagine filling a beaker with a mixture of ice and water, then placing the beaker on a hot plate and heating the mixture. Assume an even rate of heating. If you continue heating the mixture until 3 minutes after all of the ice has melted, how will the temperature of the mixture change as melting occurs? Work with your group to make as detailed a prediction as you can – think back to your experience in physical science or middle school science. Create a graph to present your hypothesis. Use “time” as the independent variable and “temperature” as the dependent variable. Label each axis, including appropriate units. Add notes to your graph to make its meaning clear to the reader (3 points). Title ________________________________________________ Y-Axis label X-Axis label Read This! Heating Curves A graph of an experiment in which heat is added to a pure substance at a steady rate is called a heating curve. Heating curves are usually produced to study how temperature changes while a pure substance is undergoing the physical changes of state from solid (s) to liquid (l), liquid to gas (g), or both. 2. Work with your group to design an experiment that will allow you to create a partial heating curve for pure water. Your curve must include the change of state from S to L and must continue for 3 minutes after all the (s) has turned to (l). Outline your procedures below. Be ready to share your experimental design with the class. You may revise your design after you have heard other groups’ ideas. Note: we will use Data Studio (3 points). 3. Conduct your experiment. Record the data you need to graph your results. 4. Graph your results, adding labels and notes to make the results understandable to the reader. Title, axes labels (with units), data runs labeled. Print your graph one graph per group and attach the graph to the group recorder’s lab. 5. Compare your results graph with your prediction graph. Describe the similarities and differences between these two graphs, citing specific pieces of evidence from # 1 and # 4 above (2 points). Similarities Differences 6. Based on your group’s partial heating curve for pure water, identify the melting point temperature for water. Describe how you reached your answer (2 points). 7. Describe how the shape of your heating curve would change if you were to start with a greater total volume of the ice/water mixture. Explain your reasoning (2 points). 8. Describe how the shape of the your heating curve would change if you were to start with the same amount of water but twice as much ice in the mixture. Explain your reasoning (2 points). 9. Use the internet to find a standard heating curve for pure water. Sketch and label the important parts of the standard heating curve (3 points). Title: Standard heating curve for pure water 10. Compare your results graph (#4) to the standard heating curve for pure water. Describe the similarities and differences (2 points). Similarities Differences 11. Based on the standard heating curve for pure water, identify the melting point of water (1 point). 12. Identify sources of error (in your experimental design, in the way you conducted the experiment, or in the way you presented your results) that could have contributed to the inaccuracies of your results. Explain how each would have affected your final graph (3 points). Read This! Changes of State or Phase Changes A solid substance melts to form a liquid when its temperature is raised high enough. The temperature at which a solid melts is characteristic of that particular pure substance. When a pure substance has melted, its molten form will turn back to solid (freeze) at the same temperature at which the solid melted. The physical form of a substance (solid, liquid, or gas) is referred to as a phase of matter or a state of matter. The changes that occur when a solid melts to liquid, a liquid freezes to solid, a liquid evaporates to gas, a gas condenses to liquid, a solid sublimates to gas, or a gas precipitates to solid are all called by the general term of phase changes or changes of state. 13. Write a descriptive paragraph that tells the complete story of what is happening from the time heat is added to ice (the solid form of water) until the time when all that remains is water vapor (the gas form of water). Use the terminology in the box above as part of your story. Cite quantitative and qualitative information as part of your story. Refer to the standard heating curve graph for pure water (5 points).