Radioactivity web
advertisement
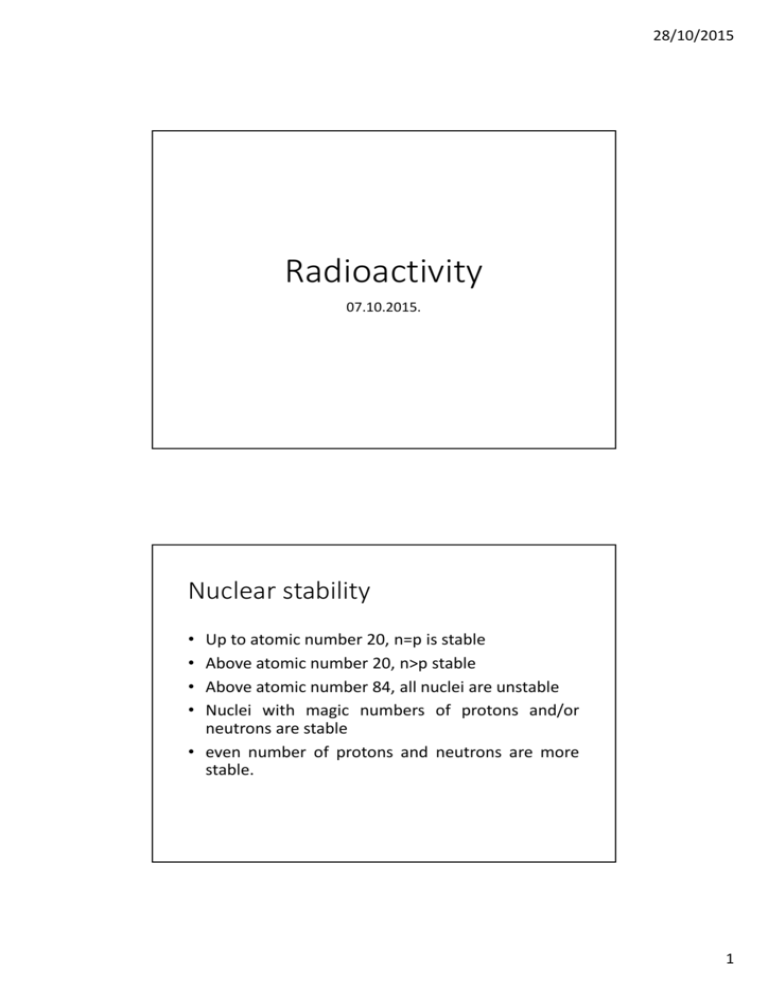
28/10/2015 Radioactivity 07.10.2015. Nuclear stability • • • • Up to atomic number 20, n=p is stable Above atomic number 20, n>p stable Above atomic number 84, all nuclei are unstable Nuclei with magic numbers of protons and/or neutrons are stable • even number of protons and neutrons are more stable. 1 28/10/2015 Radioactivity • The emission of radiation by unstable atomic nuclei undergoing radioactive decay. • Types: • Alpha-radiation • Beta-radiation • Gamma-radiation • Ionization: • energy transferred to an orbital electron to remove if from an atom • an ion pair is produced (the newly freed electron(-) and the rest of the atom(+)) • <-> excitation: energy transferred to an orbital electron to displace it further away from the nucleus. • LET (Linear Energy Transfer): the energy transferred to the medium over a certain distance (J/m, ion pairs/cm of tissue). Higher LET causes greater biological impact. Discovery of radioactivity • Antoine Henri Becquerel – 1896: radiation from uranium salts exposed films (photopaper). • Marie Currie – 1898: work with radioactive samples (discovery of polonium, radium). • The Nobel Prize in Physics 1903 • • • • Antoine Henri Becquerel 1/2 Pierre Curie 1/4 Marie Curie 1/4 "in recognition of the extraordinary services they have rendered by their joint researches on the radiation phenomena discovered by Professor Henri Becquerel". • The Nobel Prize in Chemistry 1911 "in recognition of her services to the advancement of chemistry by the discovery of the elements radium and polonium, by the isolation of radium and the study of the nature and compounds of this remarkable element". • Ernst Rutherford – 1903: discovered that alpha radiation contains helium nuclei. 2 28/10/2015 Alpha-decay • emission of a helium nucleus (alpha-particle) from a large nucleus of an atom. • He2+ ion is emitted • A (mass number) decreased by 4 • Z (atomic number) decreased by 2 • Highly ionizing – LET: 4K-9K ion pairs/µm in tissue. • range is few cm in air, < 0.5 cm in water, < 60 µm in tissue • easily shielded (e.g., paper, skin). • daughter nucleus left in excited state • the energy spectrum of alpha decay is discrete Beta radiation • • • • • • • • • High speed electron (e- = beta-particle) ejected from nucleus A (mass number) does not change Z (atomic number) decrease or increase by 1 range is a few tens of cm in air, < 2cm in water, a few mm in tissue Low LET causing light damage (6-8 ion pairs/µm in tissue) Aluminium and other light materials can be used for shielding. continuous energy spectrum X-ray can be produced (Breaking or Characteristic Radiation) e+: positron (antimatter) – annihilation (combination with an electron) produce gamma radiation 3 28/10/2015 Generating beta radiation 1 0 n→11p + + 00e − + ν ( antineutrino ) 1 1 p + →01n + 00e + + ν ( neutrino ) 137 55 22 11 − Cs→137 56 Ba + e +ν 22 Na →10 Ne + e + + ν Radioactive sodium isotope Gamma radiation • Gamma rays are photons emitted from the nucleus • Electromagnetic radiation (f>1019Hz, E>100keV (1.6*10-14J), λ<10-12m) • No mass; no charge, speed = Clight • Long range (km in air, m in body) • light damage • usually shielded with lead or concrete • radiation emitted by the atomic nucleus ↔ x-ray: radiation emitted by the atom due to electron transitions 4 28/10/2015 Radioactive Decay λ = N 1/ λ: = 2 0.693 = λ λ !" # = half-life: the time for 50% half of the atoms in a radioactive substance to disintegrate. t Radioactive Decay λ = λ: $ N 1/ %= 1 λ & = average lifetime: the length of time for 63.2% of 36.8% the atoms in a radioactive substance to disintegrate. 8225 t 5 28/10/2015 • The end! 6










