2014 Chemistry on a shoestring course description
advertisement

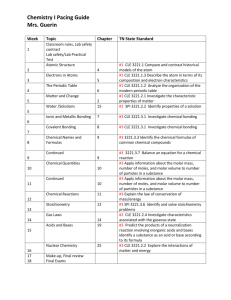
S C H O O L O F E D U C A T I O N CHEMISTRY ON A SHOESTRING: AFFORDABLE STANDARDS-­‐BASED LESSONS FOR SECONDARY SCIENCE TEACHERS June 9th -13th, 2014 Participation in this workshop is fully funded by the Middle Tennessee STEM Innovation Hub for educators in the Middle Tennessee Hub district. There will be no cost to the participant. The course is limited to 20 individuals. Please contact Mrs. Sarah Essary 615-547-1331 for course reservations. In this course, educators will participate in several standard based laboratory exercises that address standards in Chemistry I. The topics covered in the course will be the scientific method, significant figures, measurements, density, moles, limiting reagents, theoretical yield, percent yield, calorimetry, gas laws, dilutions and titrations. The development of inquiry based laboratory exercises and laboratory preparation will also be discussed. Time: 8:00am – 4:00pm. See below for additional notes. Learning Outcomes: 1. Educators will understand significant figures and will be able to make scientific measurements with the correct significant figures. (Day 1) 2. Educators will understand accuracy and precision when making measurements. (Day 1) 3. Educators will explore mole calculations, limiting reagents, percent yield and theoretical yield. (Day 2) 4. Educators will explore dilution and titration calculations and perform several types of titration experiments. (Day 4 and Day 5) 5. Educators will explore physical chemistry principles such as calorimetry, specific heat and gas law equations. (Day 3) 6. Educators will develop inquiry-­‐based laboratory experiments. (Day 2 and Day 5) 7. Educators will discuss formal scientific writing guidelines. (Day 5) S C H O O L O F E D U C A T I O N 8. Educators will learn effective pedagogy for teaching science. (Throughout the workshop) Course outline: Day 1 AM Session • • • • • Introductions: Classroom community building exercises will be conducted and the pedagogical significance of these activities will be discussed. Significant Figure Discussion: Why are significant figures important for good scientific measurements? CLE 3221, 3221.Inq.7 Accuracy and Precision Discussion: How do we determine that scientific measurements are accurate and/or precise? CLE 3221.Inq.4, 3221.Inq.12, SPI 3221 Inq.4, CLE 3221.Math.1, 3221.Math.1, 3221.Math.2 Cumberland University Laboratory Safety Guideline Discussion: Laboratory Safety at Cumberland University will be discussed. Making Measurements Laboratory Exercise, Part 1: In this laboratory exercise, educators will measure the mass of pennies. Correct use of balances will be discussed. Educators will determine the accuracy and precision of their measurements and represent their data graphically. Percent error of the mass measurements will be calculated. Experimental error will be discussed. CLE 3221.Inq.3, CLE 3221.Inq.4, 3221.Inq.5, 3221.Inq.6, 3221.Inq.7, 3221.Inq.8, 3221.Inq.9, 3221.Inq.10, 3221.Inq.11, 3221.Inq.12, 3221.Inq.13, 3221.Inq.14, SPI 3221 Inq.3, SPI 3221 Inq.4, SPI 3221 Inq.7, CLE 3221.Math.1, 3221.Math.10 PM Session • • Making Measurements Laboratory Exercise, Part 1: Continued from the AM session. Educators will evaluate their data and repeat the experiment to correct for any errors. CLE 3221.Inq.4, CLE 3221.Inq.5, CLE 3221.Inq.6, 3221.Inq.2, 3221.Inq.3, 3221.Inq.4, 3221.Inq.5, 3221.Inq.6, 3221.Inq.7, 3221.Inq.8, 3221.Inq.11, 3221.Inq.12, 3221.Inq.13, 3221.Inq.14, SPI 3221 Inq.1, SPI 3221 Inq.2, SPI 3221 Inq.4, SPI 3221 Inq.7, CLE 3221.Math.1, 3221.Math.10 Making Measurements Laboratory Exercise, Part 2: In this laboratory exercise, educators will measure the volume of liquids and determine which glassware (beaker, Erlenmeyer flask, graduated cylinder, pipette, burette) is the most accurate and/or precise. The experimental results of the cohort will be compared to determine which glassware is the most accurate and/or precise. CLE 3221.Inq.3, CLE 3221.Inq.4, CLE 3221.Inq.5, CLE 3221.Inq.6, 3221.Inq.4, 3221.Inq.5, 3221.Inq.6, S C H O O L O F E D U C A T I O N 3221.Inq.7, 3221.Inq.8, 3221.Inq.10, 3221.Inq.12, 3221.Inq.13, 3221.Inq.14, SPI 3221 Inq.3, SPI 3221 Inq.4, SPI 3221 Inq.5, SPI 3221 Inq.7 Day 2 AM Session • Density and Guided Inquiry Laboratory Discussion: Overview of the development of guided inquiry labs. CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, CLE 3221.2.1 • Density Laboratory Exercise: Educators will determine the density of known liquids and solids and will determine the accuracy and precision of their measurements. CLE 3221.Inq.3, CLE 3221.Inq.4, 3221.Inq.4, 3221.Inq.6, 3221.Inq.7, 3221.Inq.9, SPI 3221 Inq.4, CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, CLE 3221.2.1 • Guided Inquiry Density Laboratory, Part 1: Educators will design an experiment using density to determine the metal composition of a “meteorite”. CLE 3221.Inq.2, CLE 3221.Inq.3, CLE 3221.Inq.4, 3221.Inq.2, 3221.Inq.3, 3221.Inq.5, 3221.Inq.6, 3221.Inq.7, 3221.Inq.9, SPI 3221 Inq.3, SPI 3221 Inq.4, CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, CLE 3221.2.1 • Guided Inquiry Density Laboratory, Part 2: Educators will develop a process oriented guided inquiry lab for the determination of an unknown liquid using density. CLE 3221.Inq.2, CLE 3221.Inq.3, CLE 3221.Inq.4, 3221.Inq.2, 3221.Inq.3, 3221.Inq.5, 3221.Inq.6, 3221.Inq.7, 3221.Inq.9, SPI 3221 Inq.3, SPI 3221 Inq.4, CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, CLE 3221.2.1 PM Session • • Mole Calculation, Theoretical Yield, and Percent Yield Discussion: Overview of theoretical yield and percent yield. CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, 3221.Math.11, 3221.Math.12, SPI 3221.Math.4, CLE 3221.3.3, 3221.3.5 Aspirin Synthesis Laboratory: Educators will synthesize aspirin. The moles of aspirin, theoretical yield, and percent yield will be calculated from data obtain from the laboratory experiment. Limiting reagents will also be discussed. CLE 3221.Inq.3, CLE 3221.Inq.4, 3221.Inq.4, 3221.Inq.6, 3221.Inq.7, 3221.Inq.11, CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, 3221.Math.11, 3221.Math.12, SPI 3221.Math.4, CLE 3221.3.3, 3221.3.5 S C H O O L O F E D U C A T I O N Day 3 AM Session • • Calorimetry Discussion: Specific heat and enthalpy will be discussed. CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, CLE 3221.2.1, 3221.10, SPI 3221.2.5, CLE 3221.3.3, 3221.3.15 Calorimetry Laboratory: Educators will experimentally determine the specific heat of a metal using a calorimeter and determine the enthalpy of a chemical reaction. CLE 3221.Inq.3, CLE 3221.Inq.4, 3221.Inq.4, 3221.Inq.5, 3221.Inq.6, 3221.Inq.7, SPI 3221 Inq.4, CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, CLE 3221.2.1, 3221.10, SPI 3221.2.5, CLE 3221.3.3, 3221.3.15 PM Session • • Gas Law Discussion: The gas law PV=nRT will be discussed, as well as the method for collected gas over a liquid. The preparation for the gas law experiment will also be discussed. CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, CLE 3221.2.1, 3221.2.16, SPI 3221.2.7, CLE 3221.3.3, 3221.3.6 Gas Law Laboratory Experiment: Educators will determine the amount (moles) of CO2 gas evolved from an alka-­‐seltzer tablet. CLE 3221.Inq.3, CLE 3221.Inq.4, 3221.Inq.4, 3221.Inq.5, 3221.Inq.6, 3221.Inq.7, 3221.Inq.12, SPI 3221 Inq.4, CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, CLE 3221.2.1, 3221.2.16, SPI 3221.2.7, CLE 3221.3.3 , 3221.3.6 Day 4 AM Session • Dilution Discussion: Dilutions calculations will be discussed. CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, CLE 3221.2.1, SPI 3221.2.2, 3221.2.3, 3221.2.4, 3221.2.6 • Spectroscopy Discussion: Discussion of Beer’s Law and the use of spectrophotometers. CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.5, 3221.Math.6, 3221.Math.7, 3221.Math.10, SPI 3221.Math.3, CLE 3221.2.1, SPI 3221.2.2, 3221.2.3, 3221.2.4, 3221.2.6 • Determination of the Amount of Red Food Coloring in Popular Drinks Laboratory: The educators will use spectrophotometers to determine the amount of food coloring in popular drinks. A Beer’s Law plot will be developed using Baker’s Red Food coloring. CLE 3221.Inq.3, 3221.Inq.4, 3221.Inq.5, 3221.Inq.6, 3221.Inq.7, 3221.Inq.8, 3221.Inq.12, SPI 3221 Inq.4, CLE 3221.Math.1, CLE 3221.Math.2, S C H O O L O F E D U C A T I O N 3221.Math.2 3221.Math.3, 3221.Math.5, 3221.Math.6, 3221.Math.7, 3221.Math.10, SPI 3221.Math.3, CLE 3221.2.1, SPI 3221.2.2, 3221.2.3, 3221.2.4, 3221.2.6 PM Session • Titration Discussion: The basics of titration experimental method and calculations will be discussed and demonstrated. CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, 3221.Math.11, SPI 3221.Math.4, CLE 3221.3.3 • Acid Base Titration Laboratory: Educators will conduct an acid-­‐base titration laboratory exercise. Acid base principles will be discussed. CLE 3221.Inq.3, CLE 3221.Inq.4, 3221.Inq.4, 3221.Inq.5, 3221.Inq.6, 3221.Inq.7, 3221.Inq.12, SPI 3221 Inq.4, CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, 3221.Math.11, SPI 3221.Math.4, CLE 3221.3.3, SPI 3221.3.7, 3221.3.10, 3221.3.17, 3221.3.18, SPI 3221.3.6 • Precipitation Titration Laboratory: Educators will participate in a guided inquiry precipitation titration laboratory exercise. CLE 3221.Inq.2, CLE 3221.Inq.3, CLE 3221.Inq.4, 3221.Inq.3, 3221.Inq.4, 3221.Inq.5, 3221.Inq.6, 3221.Inq.7, 3221.Inq.12, SPI 3221 Inq.3, SPI 3221 Inq.4, CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, 3221.Math.11, SPI 3221.Math.4, CLE 3221.3.3, SPI 3221.3.6 Day 5 AM Session • Vitamin C Titration: Educators will determine the amount of vitamin C in a juice. The standardization of a titrant will be discussed and conducted in the laboratory. CLE 3221.Inq.3, CLE 3221.Inq.4, 3221.Inq.4, 3221.Inq.5, 3221.Inq.6, 3221.Inq.7, 3221.Inq.12, SPI 3221 Inq.4, CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7, 3221.Math.11, SPI 3221.Math.4, CLE 3221.3.3, SPI 3221.3.7, 3221.3.17, 3221.3.18, SPI 3221.3.6 PM Session • Development of an inquiry based laboratory experiment: Educators will design a hypothesis surrounding soda using one of the techniques used in the workshop. CLE 3221.Inq.2, CLE 3221.Inq.3, CLE 3221.Inq.4, 3221.Inq.2, 3221.Inq.3, 3221.Inq.5, 3221.Inq.6, 3221.Inq.7, 3221.Inq.12, SPI 3221 Inq.3, SPI 3221 Inq.4, CLE 3221.Math.1, CLE 3221.Math.2, 3221.Math.2, 3221.Math.3, 3221.Math.7 S C H O O L O F E D U C A T I O N • Formal Lab Report Discussion: The basics of a formal lab report will be discussed. CLE 3221.Inq.6, 3221.Inq.2, 3221.Inq.13, SPI 3221 Inq.5 NOTES: Candidates interested in Master’s +30 credit must apply to the university and pay all applicable admissions and per credit fees. Additional work will need to be completed for Master’s +30 credit. Candidates will enroll in ED 5875 – Chemistry on a shoestring: Affordable standards-­‐based lessons for secondary science teachers. Teachers in STEM Hub partner districts can receive a stipend of $62.50 per day upon satisfactory completion of the course. Residency in Cumberland University’s new residence hall on campus is available at a fee of $22.50 per day for the duration of the course.