Chemistry I Pacing Guide Mrs. Guerin
advertisement
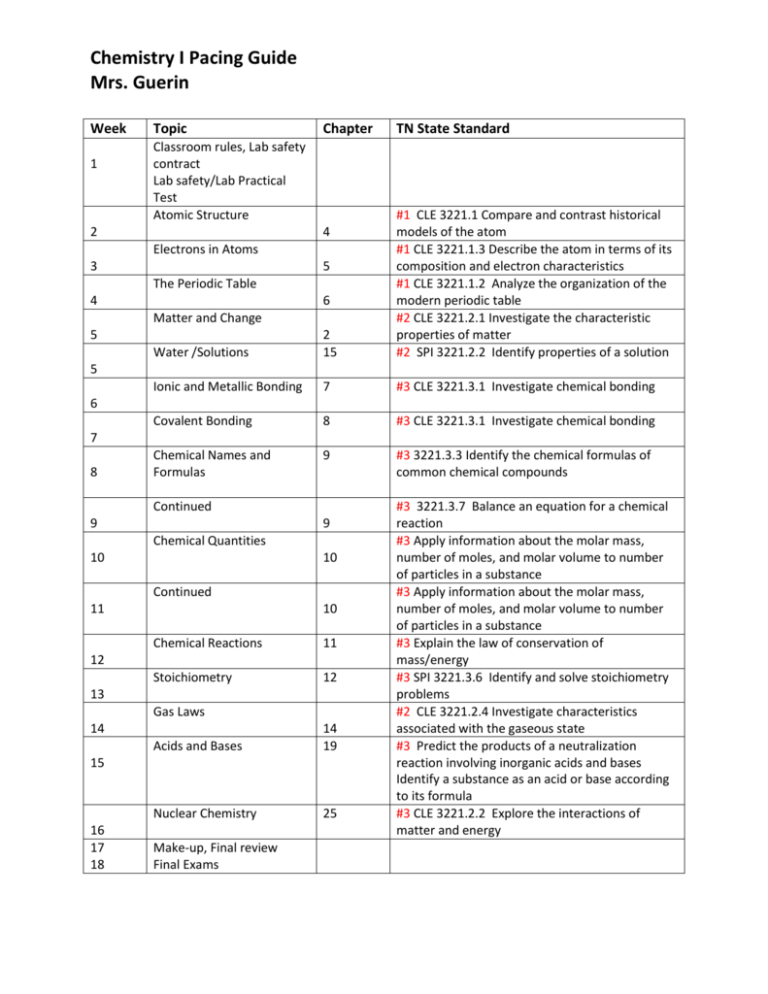
Chemistry I Pacing Guide Mrs. Guerin Week 1 Topic Chapter Classroom rules, Lab safety contract Lab safety/Lab Practical Test Atomic Structure TN State Standard Water /Solutions 2 15 #1 CLE 3221.1 Compare and contrast historical models of the atom #1 CLE 3221.1.3 Describe the atom in terms of its composition and electron characteristics #1 CLE 3221.1.2 Analyze the organization of the modern periodic table #2 CLE 3221.2.1 Investigate the characteristic properties of matter #2 SPI 3221.2.2 Identify properties of a solution Ionic and Metallic Bonding 7 #3 CLE 3221.3.1 Investigate chemical bonding Covalent Bonding 8 #3 CLE 3221.3.1 Investigate chemical bonding Chemical Names and Formulas 9 #3 3221.3.3 Identify the chemical formulas of common chemical compounds 2 4 Electrons in Atoms 3 5 The Periodic Table 4 6 Matter and Change 5 5 6 7 8 Continued 9 9 Chemical Quantities 10 10 Continued 11 10 Chemical Reactions 11 Stoichiometry 12 12 13 Gas Laws 14 Acids and Bases 14 19 Nuclear Chemistry 25 15 16 17 18 Make-up, Final review Final Exams #3 3221.3.7 Balance an equation for a chemical reaction #3 Apply information about the molar mass, number of moles, and molar volume to number of particles in a substance #3 Apply information about the molar mass, number of moles, and molar volume to number of particles in a substance #3 Explain the law of conservation of mass/energy #3 SPI 3221.3.6 Identify and solve stoichiometry problems #2 CLE 3221.2.4 Investigate characteristics associated with the gaseous state #3 Predict the products of a neutralization reaction involving inorganic acids and bases Identify a substance as an acid or base according to its formula #3 CLE 3221.2.2 Explore the interactions of matter and energy











