Supporting Information – Methods Additional Information Included in
advertisement

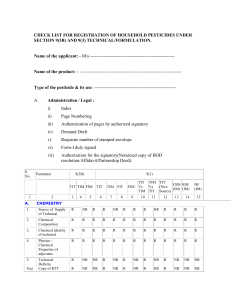
Supporting Information – Methods Additional Information Included in Database To further characterize each API, several other pieces of information were collected. The CAS (chemical abstract service) number that is associated with the parent compound was included for each API in the database. Often APIs are formulated with a carrier (e.g., hydrochloride, besylate, sodium, acetate, etc). In these cases an alternate CAS number and the name of the carrier was also included. Alternate names and/or spellings of the parent API were included if data searches resulted in relevant information under different names. To link the MaPP-FAST database with others, reference codes for www.Drugbank.ca [33] and WHO-ATC (World Health Organization Collaborating Centre for Drug Statistics – Anatomical Therapeutic Chemical codes; http://www.whocc.no/atc_ddd_index/) were included in. Finally, information on the primary therapeutic target (humans, non-human or both) was also gathered and reported. Information on physical/chemical properties and toxicity were also collected and included in the database. The chemical parameter data collected were molecular weight, log octanol:water partition coefficient (log Kow or logP), and the logarithmic acid dissociation constant (pKa). For Log P and pKa, if no measured value was available, an estimated value, calculated by structural analysis, was included. The methodology or program used for these calculations is listed with references in the database (SI-database references). Physical/chemical properties included for each API serve as a potential bridge connecting this effort with those focused on exposure. To facilitate future analyses of MaPPFAST, some of the broader classes could be further divided into sub-classes (Table SI-1). For example, the class DPRES (antidepressants) was divided into five subclasses: selective serotonin reuptake inhibitors (SSRIs), serotonin antagonist and reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors (SNRIs), tetracyclic antidepressants, and tricyclic antidepressants. These sub-divisions were largely based on molecular target differences and were derived from the same sources mentioned previously (e.g., MeSh, WHOCC-ATC, drugs.com). This subdivision was not used for our analyses described herein, but provides the database with additional flexibility for future work. Acute toxicity data for API were also collected and curated into the database. While most pharmaceuticals by design are not acutely toxic to target species [37], a property confirmed by studies with aquatic species [6], it remains important to identify those drugs where acute toxicity might contribute to potential hazard. While beyond the scope of this present work, use of acute toxicity independently or as part of a calculated metric may provide additional insight as part of a read-across strategy. Rat oral LD50 values, the most commonly available measurement, were selected to represent acute toxicity. Information included in this toxicity parameter was appropriately referenced. If a range of toxicity data were available, the lowest LD50 value was selected for inclusion. If rat toxicity values were not available, data from other rodent or mammalian species were used, and this was noted in the database. If no oral LD50 data were available, other routes of exposure (e.g., subcutaneous or intravenous injection) were utilized and similarly noted within the database. References (Numbers correspond to those in primary text: 6. Brausch JM, Connors KA, Brooks BW, Rand GM. 2012. Human pharmaceuticals in the aquatic environment: a critical review of recent toxicological studies and considerations for toxicity testing. Rev. Environ. Contam. Toxicol. 218: 1–99. 33. Law V, Knox C, Djoumbou Y, Jewison T, Guo AC, Liu Y, Maciejewski A, Arndt D, Wilson M, Neveu V, Tang A, Gabriel G, Ly C, Adamjee S, Dame ZT, Han B, Zhou Y, Wishart DS. 2014. DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res. 42: D1091-1097. 45. Hardman, J.G., Limbird, L.E. eds, 2001. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 10th ed. McGraw-Hill, New York, USA,