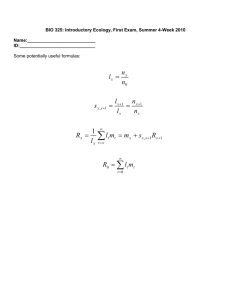
Structure-function relations in dendritic spines: Is size important?
advertisement

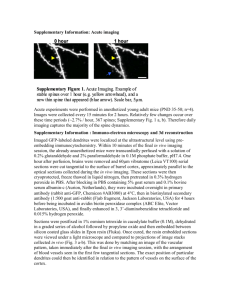
HIPPOCAMPUS 10:587–595 (2000) Structure-Function Relations in Dendritic Spines: Is Size Important? Eduard Korkotian and Menahem Segal* Department of Neurobiology, The Weizmann Institute, Rehovot, Israel ABSTRACT: The recent use of novel high-resolution imaging methods of living neurons in vitro has led to a change in the view of the dendritic spine, from a stable, long-term memory storage device to that of a dynamic structure, which can undergo fast morphological changes over periods of hours and even minutes. While the functional significance of these changes in spine dimensions is still obscure, we have obtained evidence to indicate that the length of the spine has a critical role in determining the degree of interaction between the spine head and the parent dendrite, such that longer spines are more independent of the parent dendrite than the short ones. We have now studied the role of intracellular calcium stores in determining the magnitude and time course of spine responses to a calcium surge evoked in response to glutamate, which causes an influx of calcium, and the results indicate that spine morphology has an important role in determining the involvement of the stores in calcium responses. Since spines can change their length over a rather short time, these results indicate that changes in spine length serve to fine-tune the interaction between the spine head and the parent dendrite on a continuous basis. Hippocampus 2000;10:587–595. © 2000 Wiley-Liss, Inc. KEY WORDS: development calcium stores; tissue culture; plasticity; hippocampus; INTRODUCTION The great variety of dendritic spine shapes, sizes, and density distribution on the parent dendrites of a single neuron and the apparent persistence of spines throughout the life of the neurons underlie the assertion that the spine is the unitary locus of memory formation and storage. While this intuitive view prevailed throughout the 20th century, evidence for it is rather scarce. This is due to the small size of the dendritic spine, which is prohibitive to a systematic electrophysiological analysis. Thus, it is not entirely obvious that the morphological heterogeneity seen among spines has any functional relevance. Based on EM measurements of spine dimensions, early modeling studies concluded that the spine neck cannot constitute a barrier for transfer of charge between the spine head, where the synapse is made with a presynaptic terminal, to the parent dendrite (Shepherd, 1996). More recent studies, using high spatial and temporal resolution imaging methods of spines and dendrites, concluded that the spine is actually a unique calcium Grant sponsor: US-Israel Binational Foundation; Grant number: 97/230. *Correspondence to: Menahem Segal, Department of Neurobiology, The Weizmann Institute, Rehovot, 76100 Israel. E-mail: menahem.segal@weizmann.ac.il Accepted for publication 13 June 2000 © 2000 WILEY-LISS, INC. compartment, in that an afferent stimulation that is subthreshold for generation of an action potential can evoke changes in intracellular calcium concentration ([Ca2⫹]i) that can be restricted to single spines. Synaptic stimulation, which causes release of glutamate from presynaptic nerve endings, causes activation of the postsynaptic glutamate receptors located on dendritic spines. Activation of the glutamate receptors can cause influx of calcium into the spine via several routes. The main one is probably the voltage/glutamate/glycine-gated NMDA channel (Kovalchuk et al., 2000; Segal, 1995a). Voltage-gated calcium channels, known to exist in dendritic spines (Yuste and Denk, 1995), can also be activated by the depolarization of the spine, and the possibility that the observed changes in [Ca2⫹]i result from release of calcium from stores has also been suggested (Emptage et al., 1999). Calcium stores are associated with smooth endoplasmic reticulum, which has been studied extensively in dendritic spines of hippocampal neurons by Spacek and Harris (1997). Functionally, stores can be caused to release bound calcium into the intracellular space by two distinct receptors, the ryanodine receptor and the IP-3 receptor. The former has been found in dendritic spines of cultured hippocampal neurons, and is known to be activated directly by caffeine (Hernandez-Cruz et al., 1995). Caffeine-evoked calcium transients have been described in the hippocampus (Garaschuk et al., 1997), and the machinery for caffeine-induced calcium release from dendritic spines has been studied by Korkotian and Segal (1998). Thus, the spine is a unique chemical compartment in that it may allow a local rise of [Ca2⫹]i needed for activation of plasticityrelated signaling molecules. This realization facilitated renewed interest in the role of spines in memory functions. Some of the emerging issues, still not resolved, include the functional significance of the great heterogeneity of spine morphologies, or, simply stated, do spine shape and size affect its function? We obtained preliminary data by manipulating calcium released from intracellular stores using caffeine, suggesting that spine neck length correlates with mechanisms that link calcium flux in the spine head with the parent dendrite (Korkotian and Segal, 1998; Volfovski et al., 1999). 588 KORKOTIAN AND SEGAL These studies, conducted with fast calcium imaging in a confocal laser scanning microscope, were now extended to include responses of spines and parent dendrites to glutamate. The results suggest that spine length has an important role in spine/ dendrite communication. METHODS Cultures Hippocampal cultures were prepared as described (Papa et al., 1995). Briefly, dissociated hippocampi of 19-day-old embryos were plated onto poly-l-lysine-coated 12-mm glass coverslips in Eagle’s MEM containing 10% heat-inactivated horse serum and 5% fetal calf serum. Two to three-week-old cultures were used for the imaging experiments. A coverslip was transferred into the recording chamber, and placed in a confocal laser-scanning microscope (CLSM, Leica, Heidelberg, Germany) where it was perfused with recording medium containing (in mM) NaCl 129, KCl 4, MgCl2 1, CaCl2 2, glucose 4.2, and HEPES 10; pH was adjusted to 7.4 with NaOH, and osmolarity to 320 mOsm with sucrose. The recording medium was perfused at a rate of 3–5 ml/min at room temperature. In calcium-free medium, calcium was replaced by equimolar magnesium. Drugs were prepared in the recording medium from frozen stocks before use. Glutamate (0.1 mM) was dissolved in the recording medium and loaded in a pressure pipette with a tip diameter of 2 m, which was placed some 25 m from the dendrite. Caffeine (1 mM) was applied in the perfusion medium. Presynaptic terminals were stained with the fluorescent dye FM4-64 (Molecular Probes, CA) in a medium containing 50 mM KCl, replacing equimolar NaCl, which was superfused into the culture for 1 min, followed by extensive wash in calcium-free medium. Ryanodine receptor immunoreactivity in spines was assessed as before (Korkotian and Segal, 1998). Imaging The confocal laser scanning microscope (CLSM) is equipped with an argon-ion laser for excitation at a wavelength of 488 nm, and a HeNe laser for excitation at 543 nm. Individual cells were impaled with micropipettes containing 10 mM Oregon Green-1. The dye was iontophoresed into the cell for 1 min, and was allowed to equilibrate in the cell for about 1 h before experiments commenced, to assure equal distribution of the dye in the different cellular compartments. For each experiment, a fresh spine/dendritic segment in a new dish was used. Images of 256 ⫻ 256 pixels were taken with a 63⫻ water immersion objective. Three-dimensional (3D) reconstruction of the dendrites was made from successive 0.1-m optical sections. An apparent nonuniformity in staining of the dendrites was occasionally seen due to the different number of sections used in the particular reconstructed dendrite. A line was scanned through the center of a dendrite/spine pair (about 0.8 ms per line) to reveal fast changes in fluorescence during a response to drug application (Fig. 1). Laser light was reduced to 1–3% of nominal intensity. Using this setting, we were able to stimulate the same spine/dendrite pair every 5–10 min for 2–3 h with no significant loss of reactivity due to dye bleaching or photodynamic damage. Also, baseline fluorescence did not change across the 2–3-h observation time. An effort was made to scan single lines through spine/dendrite segments having the same initial fluorescence intensity, so as to obtain similar baseline values for calculating the response value DF/F. Fluorescence was quantified using Leica analysis software and Adobe Photoshop (Adobe Systems, CA). Autofluorescence, measured in BAPTA-AM-loaded cells, under identical conditions as used here, was negligible (Korkotian and Segal, 1998). RESULTS Glutamate-Evoked Calcium Signals in Short and Long Dendritic Spines High-resolution imaging of Oregon-Green-loaded cells can easily distinguish between spines of different lengths, residing side by side on the same dendrite (Fig. 1). A brief application of glutamate near the spine/dendrite pair caused a fast-onset slow decay of [Ca2⫹]i surge, detected with the fast line scan mode of the CLSM. The exact latency of the response to glutamate could be measured in both the spine and the adjacent parent dendrite (Fig. 1A2,B2). Responses to glutamate could be evoked repetitively from the same spine/dendrite pair without fatigue for several hours. For the analysis, we grouped together spines with short necks (0.1– 0.4 m neck length) and compared them to spines with long necks (⬎0.5 m). In all the analyzed cases, it was verified, using 3D reconstruction of the spine and parent dendrite, that we have visual access to the entire length of the spine. Since we (Segal, 1995c; Korkotian and Segal, 1999b) and others (Halpain et al., 1998) have shown that exposure to glutamate can cause shrinkage of dendritic spines, we were careful to include only spines that did not change length across the experiment. In fact, in the conditions that we used in the FIGURE 1. Dendritic spines respond to glutamate by a fast rise in [Ca2ⴙ]i. A, B: Short and long spines, respectively. 1: Images of the two spine/dendrite segments, reconstructed in 3D. In each, a line was scanned between the spine head and the parent dendrite (arrows). Some fluorescence was seen through the spine neck. 2: Typical line scans depicting the beginning of the fast response to glutamate, expressed as a rise in basal fluorescence level. The image is composed of 256 lines scanned at a rate of about 0.8 ms per line, from top to bottom, going through the line seen in A, arrow, which crosses the spine head and parent dendrite. 3: Time course of the change in fluorescence seen in the region of interests comprising the spine head and the parent dendrite. Note that the spine head (light grey) of the long spine starts to increase fluorescence before the change is seen in the parent dendrite (dark grey). Figure 1 590 KORKOTIAN AND SEGAL present experiment, i.e., the absence of extracellular calcium, there were minimal if any changes in spine length following exposure to glutamate (Korkotian, unpublished observations). Significantly, there was a difference in the spine compartment, relative to the parent dendrite, between the short and long spines, in that the response to glutamate had a shorter latency in the long spines than in their parent dendrite, relative to the case of the short spines, which were similar to their parent dendrites. This distinction was evident when an averaged response of 20 short spines was compared to 18 long ones (Fig. 2). In addition, there was a clear difference in the magnitude of the responses between the short and the long spines, with the long ones expressing a larger peak response to glutamate compared to their parent dendrites, as seen before (Segal, 1995a; Korkotian and Segal, 1998). Intracellular Calcium Determines Responses to Glutamate In previous studies we explored the role of intracellular calcium stores in the calcium-handling ability of spines, and proposed that dendritic spines contain caffeine-sensitive stores (Korkotian and Segal, 1998). Since there is an unsettled debate as to the role of calcium stores in synaptically evoked calcium variations (see Kovalchuk et al., 2000; Emptage et al., 1999) we focused in the present analysis on calcium variations in response to glutamate, in the presence and absence of nominal extracellular calcium (Fig. 3). Removing extracellular calcium ([Ca2⫹]o), while leaving normal calcium levels only in the glutamate pressure pipette, caused a slow depletion of calcium stores, which maintain equilibrium with free [Ca2⫹]i, such that the remaining [Ca2⫹]i rise was due to influx of calcium in association with the applied glutamate. The removal of [Ca2⫹]o, which happened within 5 min of medium replacement, did not affect the initial response to glutamate in the short spines, indicating that calcium entering the cell via the glutamate pressure pipette is sufficient to cause a rise in [Ca2⫹]i (compare Fig. 2A to Fig. 3A, left). The fact that the calcium in the glutamate pipette was sufficient to cause a rise in [Ca2⫹]i was tested in experiments where the glutamate pipette did not contain calcium. In such cases, there was no response to glutamate in the absence of [Ca2⫹]o (data not shown). Short-term incubation with calcium-free medium was sufficient to annihilate the spine/dendrite disparity in the peak response of the long spines (compare Fig. 2B and Fig. 3A, right). The longer incubation time in [Ca2⫹]o-free medium shortened the decay time constant of recovery from glutamate response, but a substantial and stable response to glutamate was still seen (Fig. 3B). Only following exposure to a low concentration of caffeine (1 mM for 5 min) was there a marked reduction in calcium response, down to about 40% of control values (Fig. 3C). These effects are summarized in Figure 4. The rise-time of [Ca2⫹]i responses to glutamate in the control condition can be described by a sum of two exponents, with a short time constant of 90 –120 ms, and a longer time constant of 450 – 600 ms. These two time constants were characteristic of FIGURE 2. Averaged responses to glutamate of 20 short spines (A; note the symbol of short spines, here and in the following figures) and 18 long ones (B; note symbol). The averages are derived from line scans, as seen in Figure 1(3). In each of these and the following average plots, the responses in the spines are depicted in gray, and the traces of dendrites are depicted in black. Arrow indicates time of glutamate application. the long spines (Fig. 5B). The reduction in response following exposure to [Ca2⫹]o-free medium was associated with disappearance of the fast time constant of the calcium response (Fig. 5A,B). Here again, there was a large difference between the short and the long spines, with the former maintaining a similarity to the parent dendrite (Fig. 5A), whereas the long spines were progressively dissimilar from their parent dendrites (Fig. 5B). These experiments indicate that the long spines are more dependent on their own calcium stores to maintain a viable response to glutamate. The kinetic differences between the short and long spines can reflect several alternative processes. First, it is possible that the long spines are more immature than the short ones (this has been shown in young cultures, where spines are longer, on ____________________________________________ SPINE MORPHOLOGY AFFECTS SPINE FUNCTION FIGURE 3. Averaged responses to glutamate application in the absence of [Ca2ⴙ]o. Averages of 15 short spines, at left, summing 55 responses, and 16 long ones, at right, summing 43 responses, are presented, at different times after onset of perfusion with [Ca2ⴙ]ofree medium. A: 5 min after onset of o[Ca2ⴙ]o medium. B: 20 min in 591 [Ca2ⴙ]-free medium. Between B and C, cultures were superfused for 5 min with 1 mM caffeine, which facilitated the reduction in response to glutamate. Caffeine by itself did not cause a persistent change in [Ca2ⴙ]i (not shown). 592 KORKOTIAN AND SEGAL sponses to glutamate. Thus, spine morphology seems to play an important role in regulation of the link between the spine head and the parent dendrite, especially when calcium is removed, and stores are depleted. FIGURE 4. Summary diagram of the responses to glutamate at various times after perfusion with a calcium-free medium, in short and long spines and their parent dendrites. Ct, control medium with normal calcium concentration; 5ⴕ, 20ⴕ, and 40ⴕ are time in minutes after removal of [Ca2ⴙ]o. average, than in older cultured neurons; see Papa et al., 1995). If indeed they are immature, they may not be innervated by afferent fibers, and they may also contain fewer calcium stores compared to the short ones. This is not the case in the current study. First, while we did not quantify the density of innervation by presynaptic terminals, there was no distinct difference between the short and the long spines in presynaptic innervation (Fig. 6C). Likewise, although short and long spines may contain different amounts of endoplasmic reticulum (Spacek and Harris, 1997), both spine types produce similar responses to caffeine (Korkotian and Segal, 1998), and they seem to contain similar densities of calcium stores, labeled with an antibody to ryanodine receptors (Fig. 6D). Furthermore, both the short and the long spines express fast and transient spontaneous calcium fluctuations (Fig. 6A,B). These could be divided into large events, caused probably by backpropagated action potentials, and smaller events, caused by synaptic activation of the spine (Fig. 6A,B; see also Volfovsky et al., 1999). These events peaked within10 –20 ms of their spontaneous onset, and lasted about 200 – 400 ms. The spine [Ca2⫹]i changes were larger and had a faster rise time than the dendritic [Ca2⫹]i changes, as seen in the responses to glutamate. There was no apparent difference in the frequency of synaptic events, indicating that both short and long spines are likely to be innervated. Finally, it is possible that the long spines tend to lose glutamate receptors faster than the short ones in a process of desensitization (Lissin et al., 1999), regardless of the presence of calcium in stores. While this is a viable possibility, we found a rapid restoration of responses following replenishment of normal [Ca2⫹]o (data not shown), indicating that even if some desensitization occurs, it may contribute only minimally to the asymmetric reduction in re- FIGURE 5. Expanded time scale of the initial part of the response to glutamate in the short (A) and long (B) spines, taken at the beginning and end of the experiment shown in the top and bottom traces of Figure 3. For both A and B, the top two traces represent the time course at the beginning of the perfusion time, for both spines and dendrites, and the bottom two traces are the responses after 40 min of incubation with calcium-free medium. While in the short spines the time course is the same for the spines and their parent dendrite, there is a large disparity in the long spines between the spine and the dendritic calcium responses. The top traces in B were fit with a double exponent, with time constants of 90 ms and 600 ms. The bottom two traces in B were fit with a single exponent. ____________________________________________ SPINE MORPHOLOGY AFFECTS SPINE FUNCTION DISCUSSION The present results demonstrate that spine neck length may have an important role in regulating the interaction between afferent input impinging on the spine head and the parent dendrite, such that the shorter the spine neck, the higher the similarity in intracellular calcium dynamics between the spine head and the parent dendrite. This can be seen both under control conditions, and when intracellular calcium is depleted. Spine length is proposed to fine-tune the interaction between spine head and parent dendrite. The fact that both the time course of calcium rise as well as calcium clearance mechanisms are affected by spine length has been alluded to in other recent studies as well (Majewska et al., 2000). Our results do not suggest that the charge transferred by the excitatory postsynaptic potential (EPSP) is in any way affected by the length of the spine, but that subsequent, probably longer-term changes, associated with calcium-regulated “processes,” may be affected by spine length. These may include activation of kinases and phosphatases, or mobilization of glutamate receptors to the postsynaptic membrane (Lissin et al., 1999). In addition, these results suggest that stores can contribute under certain conditions to the calcium surge seen in response to the topical application of glutamate, and that the stores may have a differential effect in the short and long spines. The current results may reflect two opposite modes of involvement of calcium stores in the response to glutamate: one possibility is that when the stores are empty, influx of calcium leads to fast refilling of the stores, leading to a subsequent smaller and shorter duration of a rise of free [Ca2⫹]i. Alternatively, the increase in [Ca2⫹]i may be partly due to release of calcium from stores, in a calcium-induced calcium release process, and in the absence of calcium in the stores, the observed responses are caused only by influx of calcium, and are therefore smaller than in the control condition. While we do not present evidence to support either of these two possibilities, recent studies indicate that store antagonists block the [Ca2⫹]i transients in response to glutamate and to synaptic input (Emptage et al., 1999). Regardless of the mechanism, it is apparent that long spines are more dependent on stores than short ones, as their response to glutamate is lower than that of their short counterparts when the stores are depleted. A possible cause for the difference between the short and long spines is that the short spines contain calcium stores that are continuous with the dendritic ones, and are replenished following each response to glutamate, whereas the stores in the long ones are not fed by the parent dendrite, and thus have a limited volume which tends to deplete faster. A morphological difference in smooth endoplasmic reticulum has been described (Spacek and Harris, 1997) which may underlie this difference, except that a functional assay of the calcium stores, i.e., caffeine-induced calcium transients, does not show a distinct difference between the two (Korkotian and Segal, 1998). These results were analyzed in a model of a spine/ dendrite (Volfovsky et al., 1999), which assumes only a morphological difference between short and long spines. While [Ca2⫹]i transients in spines and dendrites have been studied extensively in recent years, there is no clear concept of what the significance is of these transients. Unlike the case of presynaptic 593 terminals, where a rise in [Ca2⫹]i is important for transmitter release, the individual postsynaptic [Ca2⫹]i transients are not assumed to play a major role in the fast depolarization caused by the incoming afferent. By comparison, [Ca2⫹]i transients are important in the dendrites, where they underlie calcium spikes, which travel to the soma and contribute to the generation of sodium action potentials. On the other hand, a sustained rise in [Ca2⫹]i has been suggested to underlie long-term processes including calcium-dependent activation of kinases and phosphatases, leading to activation/insertion/deletion of glutamate receptor subunits, proposed to underlie long-term potentiation and depression (Bear, 1995; Bliss and Collingridge, 1993). Altogether, the spine is proposed to restrict transient changes in [Ca2⫹]i, and thus protect the rest of the dendrite from the adverse effects of an excess [Ca2⫹]i (Segal, 1995b). The role of calcium stores in the regulation of calcium transients evoked by afferent stimulation has been debated recently; while one study suggests that most, if not all, of the [Ca2⫹]i change evoked by afferent stimulation is caused by release of calcium from stores (Emptage et al., 1999), another recent study could not confirm this observation and proposed, instead, that synaptically evoked calcium variations are independent of release of calcium from stores (Kovalchuk et al., 2000). In our previous work, we found that dendritic spines do contain calcium stores, which can be triggered to release calcium by caffeine. We now find that these stores are also instrumental in supporting the transient rise in [Ca2⫹]i evoked by glutamate, which causes primarily an influx of calcium from the extracellular space. Thus, in the absence of stored calcium, the calcium transient is smaller and shorter. Possible clues to functions of dendritic spines can be found in the time course of changes in spines following an environmental challenge. If indeed spines are made to store information for long periods of time, one can expect them to react slowly to environmental changes, and once they are made, to remain unchanged for a long time. This is not the case: A reduction in spine density and dendritic morphology in hibernating ground squirrels can be completely restored within 2 h of arousal from torpor (Popov et al., 1992). Likewise, changes in dendritic spine density vary quite precisely across the estrus cycle, with large variations seen over periods of 24 h (Woolley and McEwen, 1993). By the same token, cutting of brain slices can produce marked changes in spine density in less than 1 h (Kirov et al., 1999). The newly described short time constants of spine changes in vivo as well as the ability to label individual neurons and follow changes in spines in real time have popularized the use of simpler, in vitro preparations for the study of spine motility. A major advantage of the in vitro preparation is that it allows for the labeling of individual cells and for following changes in their morphology over extended periods of time. This circumvents the need to compare large independent populations, and allows a precise timing of the observed changes in dendritic spines. This ability has prompted the detection of rapid changes in spine morphology, on a minute time scale, including fluctuations around the same length (Okabe et al., 1999), and fast responses to glutamate (Segal, 1995c; Halpain et al., 1998). These fluctuations appear not to depend on action potential discharges, but are developmentally regulated, and involve rapid changes in actin polimerization (Fischer et al., 1998). Recent studies of cultured slices, which combines the advantages of in vitro cells Figure 6 ____________________________________________ SPINE MORPHOLOGY AFFECTS SPINE FUNCTION FIGURE 6. Lack of differences between short and long spines. A, B: Long and short spines, respectively (A was illustrated in Fig. 1). 1: 3-Dreconstructed images of spine-dendrite segments. 2: Summed line scans to show the responses to glutamate, left, and spontaneous events occurring later in time, at right. In both cases, the spontaneous events reflect excitatory postsynaptic potentials likely to have been evoked in the recorded spines. They do not reflect backpropagating action potentials, which are larger and longer-lasting (not shown). 3: Expansion of traces indicated in 2 by asterisks to illustrate the time course of spontaneous events. C: Image of Calcium Green-labeled dendrites and spines (green) and FM4-64-labeled presynaptic varicosities (red). Note that both the long spine (left) and the short spine (right) possess a presynaptic terminal. Several other presynaptic terminals are seen attached to the dendrite, and probably to other dendrites in the field. D: Short (top) and long (bottom) spines stained with an antibody for the ryanodine receptor (from the experiment detailed in Korkotian and Segal, 1998). d, dendrite. Scale bars in B–D, 2 m. (follow-up of individual spines) with the advantages of in vivo cells (spatial organization of a cell and its afferents) have already yielded preliminary observations of novel spines produced following expression of LTP in pyramidal neurons (Engert and Bonhoeffer, 1999) as well as rapid growth of filopodia in response to electrical stimulation (Maletic-Savatic et al., 1999). In the dissociated culture, formation of novel spines in response to release of calcium from stores (Korkotian and Segal, 1999a) or the retraction/extension of existing spines in response to glutamate (Korkotian and Segal, 1999b) have also been reported. These results, obtained primarily with in vitro preparations, paint a very different picture of spine motility and stability than ever before appreciated. The spine appears as a dynamic structure, which can change continuously as a function of synaptic input. The current reliance on in vitro systems may bias the view of the role of spines in long-term neuronal functions; after all, most of the studies cited herein deal with the immature brain, and may not reflect processes that take place in the adult one. Thus, rapid motility may be a process by which a spine searches and finds its target, but this motility may be lost weeks or months after the connection is stable. Given these limitations, it appears that the demand that changes the spine at such a fast rate has to do with the need to restrict changes in [Ca2⫹]i in the area of the activated synapse. This indicates that spine shape may have an important role in regulating the interaction between the synapse and the parent dendrite, and the ability of the dendrite to control the efficacy of the synapse and its modifiability. Acknowledgments We thank Ms. V. Greenberger for preparation of cultures. REFERENCES Bear M. 1995. Mechanism for a sliding synaptic modification threshold. Neuron 15:1– 4. Bliss TVP, Collingridge GL. 1993. A synaptic model of memory: long term potentiation in the hippocampus. Nature 361:31–39. Emptage N, Bliss TV, Fine A. 1999. Single synaptic events evoke NMDA receptor mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron 22:115–124. 595 Engert F, Bonhoeffer T. 1999. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399:66 –70. Fischer M, Kaech S, Knutti D, Matus A. 1998. Rapid actin-based plasticity in dendritic spines. Neuron 20:847– 854. Garaschuck O, Yaari Y, Konnerth A. 1997. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurons. J Physiol [Lond] 502:13–30. Halpain S, Hipolito A, Saffer L. 1998. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci 18:9835–9844. Hernandez-Cruz A, Diaz-Munoz M, Gomez-Chavarin M, Canedo-Merino R, Protti DA, Escobar AL, Sierralta J, Suarez-Isla BA. 1995 Properties of the ryanodine-sensitive release channels that underlie caffeine induced Ca2⫹ mobilization from intracellular stores in mammalian sympathetic neurons. Eur J Neurosci 7:1684 –1699. Kirov SA, Sorra KE, Harris KM. 1999. Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. J Neurosci 19:2876 –2886. Korkotian E, Segal M. 1998. Fast confocal imaging of calcium released from stores in dendritic spines. Eur J Neurosci 10:2076 –2084. Korkotian E, Segal M. 1999a. Release of calcium from stores alters the morphology of dendritic spines in cultured hippocampal neurons. Proc Natl Acad Sci USA 96:12068 –12072. Korkotian E, Segal M. 1999b. Bidirectional regulation of dendritic spine dimensions by glutamate receptors Neuroreport 10:2875–2877. Kovalchuk Y, Eilers J, Lisman J, Konnerth A. 2000. NMDA receptormediated subthreshold Ca2⫹ signas in spines of hippocampal neurons. J Neurosci 20:1791–1799. Lissin DV, Carroll RC, Nicoll RA, Malenka RC, von Zastrow M. 1999. Rapid, activation-induced redistribution of ionotropic glutamate receptors in cultured hippocampal neurons. J Neurosci 19:1263–1272. Majewska A, Brown E, Ross J, Yuste R. 2000. Mechanisms of calcium decay kinetics in hippocampal spines: role of spine calcium pumps and calcium diffusion through the spine neck in biochemical compartmentalization. J Neurosci 20:1722–1734. Maletic-Savatic M, Malinow R, Svoboda K. 1999. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283:1923–1927. Okabe S, Kim HD, Miwa A, Kuriu T, Okado H. 1999. Continual remodeling of postsynaptic density and its regulation by synaptic activity. Nat Neurosci 2:804 – 811. Papa M, Bundman MC, Greenberger V, Segal M. 1995. Morphological analysis of the development of dendritic spines in primary cultures of hippocampal neurons. J Neurosci 15:1–11. Popov VI, Bocharova LS, Bragin AG. 1992. Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation. Neuroscience 48:45–51. Segal M. 1995a. Imaging of calcium variations in dendritic spines of cultured hippocampal neurons. J Physiol [Lond] 486, 285–296. Segal M. 1995b. Dendritic spines for neuroprotection. Trends Neurosci 11:468 – 471. Segal M. 1995c. Morphological alternations in dendritic spines of rat hippocampal neurons exposed to NMDA. Neurosci Lett 193:73–75. Shepherd GM. 1996. The dendritic spine: a multifunctional integrative unit. J Neurophysiol 75:2197–2210. Spacek J, Harris KM. 1997 Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci 17:190 –203. Volfovsky N, Parnas H, Segal M, Korkotian E. 1999. Geometry of dendritic spines affects calcium dynamics In hippocampal neurons: theory and experiments. J Neurophysiol 82:450 – 462. Woolley CS, McEwen BS. 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336:293–306. Yuste R, Denk W. 1995. Dendritic spines as basic functional units of neuronal integration. Nature 375:682– 684.