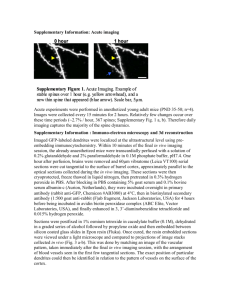
Northern blotting
advertisement

Supplementary Methods Northern blotting For Northern blots, 30 g of total RNA was resolved on 15% urea/polyacrylamide gels and transferred to Hybond N+ membrane (Amersham). RNA was immobilized by UV cross-linking (1000 J) followed by baking for 30 min at 80° C. Membranes were prehybridized in hybridization buffer (5xSSC, 20 mM Na2HPO4 pH 7.2, 7% SDS, 2x Denhardt’s solution) for at least 2 h at 50° C. Small RNAs were detected using P32 end-labeled antisense DNA oligonucleotides in hybridization buffer overnight. Membranes were washed 4x in non-stringent wash solution (3x SSC, 25 mM NaH2PO4 pH 7.5, 5%SDS, 10x Denhardt’s solution) followed by one wash in stringent wash solution (1xSSC, 1%SDS). Sealed blots were exposed to phosphorimager plates. Spine analysis Neurons were selected in a blinded manner based on eGFP staining at a low resolution at which spines were not visible. Seven consecutive optical sections of dendrites were taken at 0.45m interval, 1024x1024 pixel resolution, averaged four times, scan speed 15s per section on a Zeiss LSM510 Meta confocal microscope. Projection images were constructed from individual z-stacks and used for spine analysis by MetaMorph software. All dendritic protrusions longer than 0.4m (“spines” and “filopodia”) were considered for this analysis. Spine length was determined manually by measuring the maximal length of a line from the dendritic shaft to the outermost part of the spine head. For the determination of spine width, a line was drawn across the widest part of the dendritic protrusion (“spine head”) and the average pixel intensity of the GFP channel was derived along this line. Spine width was defined as the length of the line where pixel intensities were above an arbitrarily chosen threshold equal to double the background fluorescence. Spine volume was measured in principal as reported previously, assuming free diffusion of eGFP throughout the dendritic tree 40. Briefly, regions of interest (ROIs) were drawn around spines in the projection image and nearby dendrites. The average fluorescence of pixels within this region was measured and corrected for background fluorescence. Finally, a s/d ratio was calculated for each spine by dividing the corrected pixel intensity of the spine (s) ROI by that of the dendrite (d) ROI, thereby correcting for possible fluctuations in the filling of individual dendrites. The calculated s/d ratio represents a relative measure of spine volume. For each experimental condition, at least 600 individual spines from 15 neurons of three independent experiments were analyzed. For the calculation of standard errors, measurements from experiments performed on different days were treated as independent measurements. Statistical significance, if not otherwise stated, was assessed with paired Student’s t-test. Co-localization studies For the determination of co-localization coefficients, ROIs were drawn around randomly chosen dendrites and the degree of co-localization between red and green channel was calculated using the LSM510 co-localization tool. Data was analyzed from three independent experiments, five neurons per condition and at least three individual dendrites per neuron. MicroRNA sensor assay Cortical neurons (4DIV) were transfected with eGFP reporter constructs that contain either a perfect miR-134 binding site (“wt sensor’) or a mismatch sequence (“mut sensor”) in the 3’UTR, together with miR-134 expression vector or 2’O-Me-oligonucleotides where indicated. DsRed was co-transfected to visualize transfected neurons. 48 h after transfection, samples were processed for fluorescence microscopy. High miR-134 activity within neurons is indicated by cleavage of the wt sensor resulting in the disappearance of the GFP signal. Conversely, inhibition of miR-134 activity results in the reappearance of the GFP signal. Gelshift assay In vitro transcribed Limk1 RNA (100 ng) was incubated with 32P end-labeled microRNAs (30.000 dpm/l) as described46, and the complexes were resolved on a native 15% polyacrylamide/ 0.5x TBE gel. RNA and DNA oligonucleotides In situ hybridization: Mmu-miR-134 (LNA): cccctctggtcaaccagtcaca Mmu-miR-134 MISMATCH (LNA): tccctctggtcaaggattccga Limk1-SP6 (sense): atttaggtgacactatagaataggccctcttttgtaaagctgg Limk1-T7 (antisense): taatacgactcactatagggagagaactcagagggcagatgcac GFP-SP6: atttaggtgacactatagaataccatggtgagcaagggcgag GFP-T7: aatacgactcactatagggagatacttgtacagctcgtccatgcc Synthetic RNA oligos: MiR-134 sense: 5’Phospho-ugugacugguugaccagaggga MiR-134 as: 5’Phospho-ccucuggucaaccaguuauacu Let-7c sense: 5’Phospho-ugagguaguagguuguaugguu Let-7c as: 5’-Phospho-ccauacaaccuacuacuuuaaa 2’O-methylated oligos: 2’OMe134: ucucucccucuggucaaccagucacaaggcu 2’OMe-control: caucacguacgcggaauacuucgaaaugacc 2’OMe-let7: ucuucacuauacaaccuacuaccucaaccuu Real-time PCR: MiR134 precursor fw: gggtgtgtgactggttgacca MiR134 precursor bw: gggttggtgactaggtggcc Firefly Luc fw: tgaccgcctgaagtctctga Firefly Luc bw: tggagcaagatggattccaat Renilla fw: ggtgaagttcgtcgtccaaca Renilla bw: tgtacaacgtcaggtttaccacct Limk1 fw: cctccgagtggtttgtcga Limk1 bw: caacacctccccatggatg Beta3tubulin fw: ccccagggctcaagatgtc Beta3tubulin bw: cgcttgaacagctcctggat Cloning and Mutagenesis: MiR134 precursor fw: cagtgaattcccaaccttggtgaggcagctg MiR134 precursor bw: cagtgaattctcctggtccactgagcaggc Limk1UTR-fw: gtatccatcgatactctggagaatagaccctcaccag Limk1UTR-bw: atgctctagagggagcacagaattgattttattgag Limk1UTR miR134site-fw: tgcctctggcccccatctagaaaggctgcacacac Limk1UTR miR134site-bw: gtgtgtgcagcctttctagatgggggccagaggca MiR134 sensor fw: ggcctccctctggtcaaccagtcacaggtacct MiR134 sensor bw: cgccaggtacctgtgactggttgaccagaggga Northern probes: U6: gcaggggccatgctaatcttctctgtatcg MiR134: tcccctctggtcaaccagtcaca MiR124a: tggcattcaccgcgtgccaatt