Na2S2O3 Standardization: Lab Protocol
advertisement
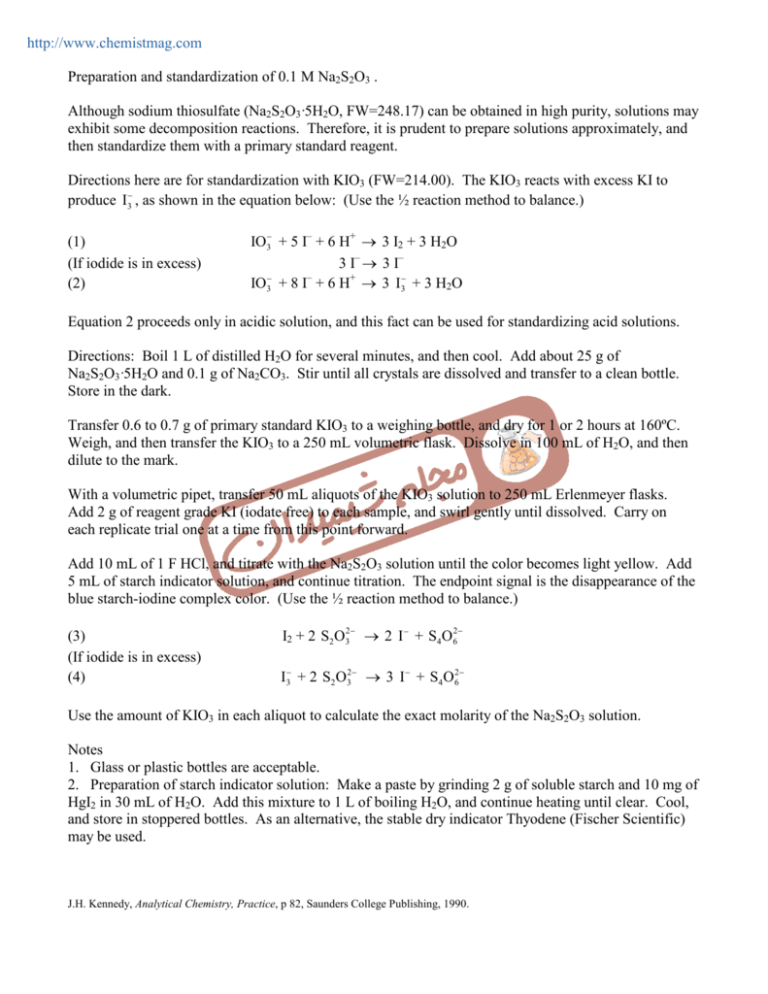
http://www.chemistmag.com Preparation and standardization of 0.1 M Na2S2O3 . Although sodium thiosulfate (Na2S2O3·5H2O, FW=248.17) can be obtained in high purity, solutions may exhibit some decomposition reactions. Therefore, it is prudent to prepare solutions approximately, and then standardize them with a primary standard reagent. Directions here are for standardization with KIO3 (FW=214.00). The KIO3 reacts with excess KI to produce I3− , as shown in the equation below: (Use the ½ reaction method to balance.) (1) (If iodide is in excess) (2) IO3− + 5 I– + 6 H+ → 3 I2 + 3 H2O 3 I– → 3 I– IO3− + 8 I– + 6 H+ → 3 I3− + 3 H2O Equation 2 proceeds only in acidic solution, and this fact can be used for standardizing acid solutions. Directions: Boil 1 L of distilled H2O for several minutes, and then cool. Add about 25 g of Na2S2O3·5H2O and 0.1 g of Na2CO3. Stir until all crystals are dissolved and transfer to a clean bottle. Store in the dark. Transfer 0.6 to 0.7 g of primary standard KIO3 to a weighing bottle, and dry for 1 or 2 hours at 160ºC. Weigh, and then transfer the KIO3 to a 250 mL volumetric flask. Dissolve in 100 mL of H2O, and then dilute to the mark. With a volumetric pipet, transfer 50 mL aliquots of the KIO3 solution to 250 mL Erlenmeyer flasks. Add 2 g of reagent grade KI (iodate free) to each sample, and swirl gently until dissolved. Carry on each replicate trial one at a time from this point forward. Add 10 mL of 1 F HCl, and titrate with the Na2S2O3 solution until the color becomes light yellow. Add 5 mL of starch indicator solution, and continue titration. The endpoint signal is the disappearance of the blue starch-iodine complex color. (Use the ½ reaction method to balance.) (3) (If iodide is in excess) (4) I2 + 2 S2 O32− → 2 I − + S4 O62− I3− + 2 S2 O32− → 3 I − + S4 O62− Use the amount of KIO3 in each aliquot to calculate the exact molarity of the Na2S2O3 solution. Notes 1. Glass or plastic bottles are acceptable. 2. Preparation of starch indicator solution: Make a paste by grinding 2 g of soluble starch and 10 mg of HgI2 in 30 mL of H2O. Add this mixture to 1 L of boiling H2O, and continue heating until clear. Cool, and store in stoppered bottles. As an alternative, the stable dry indicator Thyodene (Fischer Scientific) may be used. J.H. Kennedy, Analytical Chemistry, Practice, p 82, Saunders College Publishing, 1990.











