Solutions Lab
advertisement

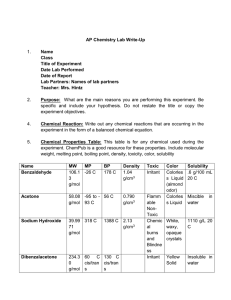
Solutions Lab We will have a solutions lab on Monday since Friday is a pep-rally schedule. I have the procedure listed below. This is all that I am giving you. You have to come up the purpose of this lab based on the procedure. Be sure to include everything for the final write up in your lab notebook. Part 1: 1. Pipet 1.0 mL of water into a test tube. 2. Mass 5.0 g of Na2S2O3 on to weighing paper. 3. From the 5.0 g weighed out, begin adding sodium thiosulfate to the water one crystal at a time. 4. Observe the ease or difficulty of getting the crystals to dissolve as the solution approaches saturation. 5. Continue to add crystals one at a time and shake until no more crystals will dissolve. 6. Solubility is based on how many grams of the solute will dissolve in 100 g of water. Since 1 gram of water equals one mL of water, what is the approximate solubility of Na2S2O3 at room temp? Part 2: 1. Pipet one mL of water into a test tube. 2. Mass 7.50 g of Na2S2O3 and add it all to the water. 3. Dissolve the crystals in the water by heating the solution gently over a Bunsen burner until the solution is clear. 4. Place the test tube in a beaker of cold water for about 10 minutes. 5. Remove the test tube from the cold water and gently tap the side with a pencil. 6. If nothing happens, drop one crystal of Na2S2O3 in the solutions but be ready because this works very quickly. Record all observations. 7. Reheat the test tube for disposal down the drain with lots of water.