In-Class Worksheet Bonding & Lewis Structures
advertisement

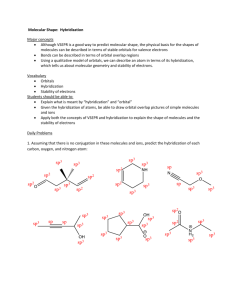
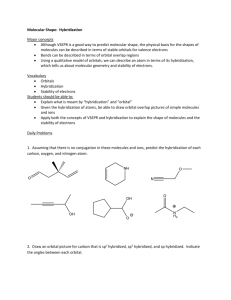
Chem201A Dr. Baxley Geometries and Valence Bond Theory Worksheet Also do Chapter 10 textbook problems: 33, 35, 47, 49, 51, 55, 57, 61, 63, 67, 83, 87. 1. Fill in the tables below for each of the species shown. a) CCl2H2 Lewis Structure, complete with formal charges (show any possible resonance structures too!) Molecular Geometry: Bond Angle(s): Polar or not? Hybridization Orbital Diagram of central atom alone Orbital Diagram of central atom in compound b) ClNO Lewis Structure, complete with formal charges (show any possible resonance structures too!) Molecular Geometry: Bond Angle(s): Polar or not? Hybridization Orbital Diagram of central atom alone Orbital Diagram of central atom in compound Chem201A Dr. Baxley c) PF6− Lewis Structure, complete with formal charges (show any possible resonance structures too!) Molecular Geometry: Bond Angle(s): Polar or not? Hybridization Orbital Diagram of central atom alone (P–): add one electron Orbital Diagram of central atom in compound d) TeCl2Br2 Lewis Structure, complete with formal charges (show any possible resonance structures too!) Molecular Geometry: Bond Angle(s): Polar or not? Hybridization Orbital Diagram of central atom alone Orbital Diagram of central atom in compound Chem201A e) NO2− Lewis Structure, complete with formal charges (show any possible resonance structures too!) Dr. Baxley Molecular Geometry: Bond Angle(s): Polar or not? Hybridization Orbital Diagram of central atom alone Orbital Diagram of central atom in compound 2. a. Draw the Lewis structure of acetone, (CH3)2CO (C in the middle, with two C’s and an O bonded to it). What is the hybridization on each carbon atom in the molecule? b. On the Lewis structure, identify the type of each bond (σ or π). c. Describe the orbital location, including type of bond, of each pair of electrons in the molecule including lone pairs (for example, there are six pairs of electrons in six different σ bonds formed between an s orbital on a hydrogen and an sp3 hybrid orbital in a carbon). Chem201A Dr. Baxley 3. Draw the Lewis structure and determine the hybridization on the central atom in each of the following molecules. Then draw the orbital diagrams of the central atom alone, and the central atom in the molecule. a. BrF3 b. XeF4 c. HCN 4. Epinephrine, also called adrenaline, is a hormone and a neurotransmitter that, among other things, is involved in the “fight or flight” response. The structure of epinephrine, with some atoms labeled with numbers, is shown below. This representation does not show any lone pairs of electrons, but all oxygen atoms with two bonds should have two lone pairs and nitrogen atoms with three bonds should have one lone pair. This structure also does not show all bonds. For the oxygen atom labeled 1, it has one bond to carbon #2 and one bond to hydrogen. The carbon labeled number 5 has one bond to nitrogen #4 and three bonds to three hydrogen atoms. a. For each of the labeled atoms, identify the shape around that atom, the bond angle, and the hybridization on that atom. b. How many π bonds are in this structure? Chem201A Dr. Baxley Answer Key 1. a. molecular geometry: tetrahedral bond angle: 109.5º polar sp3 hybridized C alone Cl H C Cl H C in CCl2H2 2p sp3 2s b. molecular geometry: bent bond angle: <120º polar sp2 hybridized N alone Cl N O N in ClNO 2p 2p sp2 2s c. molecular geometry: octahedral bond angle: 90º nonpolar sp3d2 hybridized Chem201A Dr. Baxley P– alone P– in PF6– 3d 3d 3p sp3d2 3s d. molecular geometry: seew saw (Br atoms are larger, so they will be in equatorial positions) bond angle: <90º and <120º polar sp3d hybridized e. Te alone Te in TeCl2Br2 5d 5d 5p sp3d 5s f. electron pair geometry: trigonal planar molecular geometry: bent bond angle: <120º polar sp2 hybridized O N O N alone O N O N in NO2– 2p 2p 2s sp2 Chem201A 2. Dr. Baxley sp3 sp3 H H O H C C C H H H sp2 There are six pairs of electrons in six different σ bonds formed between an s orbital on a hydrogen and an sp3 hybrid orbital in a carbon. There are two pairs of electrons in two bonds formed between an sp3 hybrid orbitals on the outside carbons and an sp2 hybrid orbital on the central carbon. There is one pair of electrons in a σ bond between an sp2 hybrid orbital on the central carbon and an sp2 hybrid orbital on the oxygen. There is one pair of electrons in a π bond between the 2p orbital on the central carbon and the 2p orbital of the oxygen. There are two lone pairs of electrons in sp2 hybid orbitals of the oxygen. 3. a. Lewis structure is below. Br is sp3d hybridized. Orbital diagrams are below. Br alone 4d 4p Br in BrF3 4d sp3d 4s b. Lewis structure is below. Xe is sp3d2 hybridized. Orbital diagrams are below. Chem201A Dr. Baxley Xe alone Xe in XeF4 5d 5d 5p sp3d 5s c. Lewis structure is below. C is sp2 hybridized. Orbital diagrams are below. C alone C in HCN 2p 2p sp 2s 4. O1: bent, <109.5º, sp3 C2: trigonal planar, 120º, sp2 C3: tetrahedral, 109.5º, sp3 N4: trigonal pyramidal, <109.5º, sp3 C5: tetrahedral, 109.5º, sp3