Diels-Alder Reactionn
advertisement

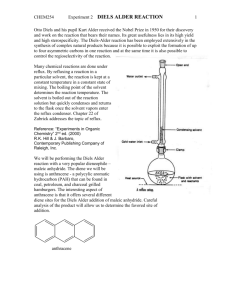
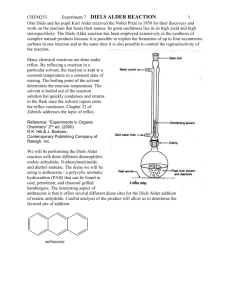
Experiment: Diels-Alder Reaction Diels-Alder cycloaddition reactions have proven to be quite useful routes to form new sixmembered ring structures. They are also intriguing reactions because of their concerted, cyclic mechanisms with six π-electrons moving at once to generate the new ring structure. They also are noted for their stereospecificity (syn, endo) and seemingly curious electronic requirements. Much of this chemical behavior can be explained by a closer look at Molecular Orbital Theory, which is covered in your textbook. If you have already covered the Diels-Alder reaction in lecture, you will recall that the reaction requires a conjugated diene and a “dienophile”. There are some additional requirements to make the reaction proceed smoothly. First, it is essential that the diene be able to achieve the s-cis conformation in order for the π-bonds to be oriented for overlap with the dienophile. s-trans s-cis This molecule can rotate between s-trans and s-cis orientations. Only the s-cis conformation reacts. This molecule is "locked" into the s-cis orientation. This molecule cannot rotate into the required s-cis orientation. The molecule on the left, 1,3-butadiene, is free to rotate about the middle C-C bond, allowing it to achieve the correct s-cis orientation for a Diels-Alder reaction. As the s-cis conformation reacts, the equilibrium shifts to replace it. The center molecule, 1,3-cyclohexadiene, would be expected to react rapidly as a result of its fixed s-cis orientation of the double bonds. The molecule on the right, however, is “locked” in the wrong conformation, the s-trans orientation, which does not allow correct overlap of the p-orbitals, therefore, no Diels-Alder reaction would be expected with this molecule. The second requirement for a facile Diels-Alder reaction involves the dienophile. Diels-Alder reactions proceed with less difficulty if the dienophile has electron withdrawing groups present. O O H H H O Increasing dienophile reactivity When these conditions are met, refluxing the diene and dienophile is sufficient to make the reaction proceed to form a new six membered ring. In this experiment, you will heat anthracene with maleic anhydride to form the Diels-Alder bicyclic-adduct shown below. The bold-lines show the new six-membered ring that is formed by the Diels-Alder reaction. O O O O O + O anthracene maleic anhydride Diels-Alder Adduct Here, anthracene is acting as the conjugated diene. As you can see from the structure of anthracene above, these cyclic systems “lock” the double bonds in the s-cis orientation required for this reaction. You might also have noticed, however, that anthracene is not only conjugated, it is aromatic. Generally, aromatic compounds with a single ring, such as benzene-based compounds, are unreactive toward dienophiles. The loss of aromaticity makes the reaction too unfavorable. Polycyclics such as anthracene, however, can be driven to react using higher temperatures. Only a portion of the total aromaticity is lost (two aromatic rings remain) and in such systems, the loss of aromaticity of one ring of a polycyclic is lower than the amount of energy lost when an isolated benzene ring loses aromaticity. It is also important to note that as the p-orbitals begin to overlap, the transition state of Diels-Alder reactions also has aromatic character due its cyclic nature and to the 6 π electrons involved. This aromatic character helps to lower the energy of the transition state of Diels-Alder reactions. Overall, then, for anthracene it is sufficient to use a high boiling solvent such as xylene to drive the reaction forward. We can also help push the reaction forward by using a highly activated dienophile, maleic anhydride, which has two strong electron withdrawing groups. Note: Looking at the reaction as drawn above, you can see that the anhydride adds to the center ring of anthracene. Based on the structure of anthracene, however, it also seems possible for the anhydride to react with one of the side aromatic rings. O O O O O + X O anthracene maleic anhydride This reaction, shown above, does not occur to any significant extent, however, because the product formed has less aromatic stabilization. Testing has shown that products with two separate benzene rings are more stable than structures with a naphthalene component. In other words, the aromatic stabilization of naphthalene is not double the aromatic stabilization of a single benzene ring, but rather it is somewhat lower. Addition to the center ring, then, produces a more stable product with two benzene rings, via a more stable transition state. As a result, the product labeled as the “Diels-Alder Adduct” in the first reaction shown is the predominant product. The two starting materials are solids, which can be weighed easily and added to a round bottom flask. Xylene will be used as a solvent to give a higher reflux temperature. The reflux apparatus and all reagents must be absolutely dry to prevent the maleic anhydride from reacting with water to form the dicarboxylic acid, maleic acid. You will be using a drying tube at the top of the reflux condenser to maintain dry conditions throughout the reflux. Remember, you begin timing the reflux when you can see a ring of condensing solvent in the reflux condenser. Make certain that you reflux the entire hour to ensure that sufficient product has formed. As the reaction progresses, you should notice a color change in the solution; record this in your observations. Once the reaction is complete, it is important to cool very slowly to allow pure product to crystallize. This step is like running a recrystallization in your round bottom flask immediately after the reaction is complete. So patience is important if you want to obtain pure product. Do not use ice to cool at any point or you will co-crystallize any remaining anthracene, which is only slightly soluble in cool xylene. This experiment should be fun to run as the procedure is straight-forward and the product is easy to isolate. You might notice that anthracene and your product appear slightly bluish. Take a look at them under ultra-violet light and you will see that they both fluoresce. Pre-lab Preparation Before coming to lab, write the following assignment in your lab notebook. 1. Review the chemistry of Diels-Alder reactions. 2. In tabular form write the physical constants (boiling point and melting point) for anthracene, maleic anhydride, ethyl acetate and all three xylenes. You will be using “xylenes” as a solvent, which is a lower cost mixture of all three isomers. Look up the information for all three xylenes and include them in your table. 3. Answer the following questions regarding the experimental procedure. a. Why is dry glassware necessary for this Diels-Alder reaction? What side reaction would occur if water were present? b. Why should the solid product be washed with ethyl acetate instead of cold xylene? Experimental Procedure ! Safety Considerations ! Anthracene and your organic product can be harmful if ingested. Use gloves and wash your hands at the conclusion of lab. ! Maleic anhydride is corrosive. If any spills on your skin, rinse thoroughly with water and then wash the affected area with soap and water. ! Xylene is harmful if ingested. Use gloves and work in the hood. ! Dispose of all organic waste in the containers provided. 1. Place a 50-mL round bottom flask onto a heating mantle equipped with a calcium chloride drying tube. Warm the round bottom to drive off any water which may be weakly bound to the surface. Allow the flask to cool to a comfortable handling temperature with the drying tube in place. 2. To the round bottom flask, add 1.2 g of anthracene and 0.67 g of maleic anhydride (preweighed for you in vials). After adding both of these solids, add 15 mL of xylene. Weigh a few boiling stones and add these to the flask. (The crystals form on the stones making them difficult to remove. By weighing them at the start, you can simply weigh your product with the stones included and subtract the mass of the stones to determine the mass of your product.) 3. Attach a reflux condenser onto the flask and then place the drying tube on the top of the reflux condenser to keep water out of your reaction. Begin to heat on a fairly high setting to bring the xylene quickly to reflux. Continue to reflux for one hour, while noting any color changes. 4. After the reflux time is complete, you may notice that some crystals have formed on your boiling stones. This is your product. Allow the round bottom flask to cool to room temperature. More crystals of product should form as the reaction cools. Do not cool on an ice bath as this may cause any unreacted anthracene to precipitate. 5. Filter the product using a Hirsch funnel and vacuum filtration. Use a spatula to scrape out as many crystals as possible. 6. Once you have finished filtering, wash the product with two small portions of cold ethyl acetate to wash away impurities and to help to remove the higher boiling xylene. If there are crystals remaining in the flask, rinse the flask with these ethyl acetate wastes before pouring them into the funnel. 7. Transfer the crystals to a piece of tared filter paper and allow to dry at least 24 hours before weighing and taking a melting point. When you take a melting point, you might also want to obtain a melting point of pure anthracene at the same time for comparison. Both are high melting solids. Prepare a capillary tube with anthracene in it and set it aside with your product until you are ready to test both. 8. To confirm that the reaction has proceeded, observe both the starting anthracene and your product under ultraviolet light. Note any differences between the starting material and product in your observations. 9. After weighing, place your product into a vial and label with your name, product weight and melting point. Be prepared to turn this product in to your lab instructor with your lab report. Post-Lab and Report Requirements For this report, include answers to the following four questions. 1. Calculate the moles of all reactants and then calculate your theoretical yield and percentage yield for this experiment. 2. Draw the structures shown below and indicate whether each would work in a DielsAlder Reaction. For each, give your reasoning. Be specific. O 3. For each of the following, draw the expected Diels-Alder product(s). F a) b) + F O + O CH3