Isolation of Natural Products Natural Products - TAMU
advertisement

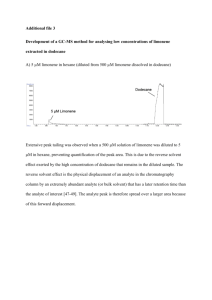
11/30/2012 Isolation of Natural Products And Some Reactions of Alkenes Natural Products A natural product is an organic chemical that has been isolated and purified from a living organism (plant or animal). Compounds that are part of common metabolic pathways (primary metabolism) for plants and animals are generally excluded. Natural products are considered products of secondary metabolism – metabolism not directly necessary for growth and survival and are often unique to a family of organisms. 1 11/30/2012 Natural Products Many natural products are of interest because of their biological activity in other organisms, including humans. Many natural products are quite toxic to animals and humans. Natural does not mean ‘safe’. Natural Products from Plants Plants provide the largest number of known natural products. Their utility to the plant ranges from reproduction (fragrances that attract bees for pollination) to protection (substances that repel animals or insects). Isolation of a specific natural product requires separation from polymeric materials (cellulose, DNA, etc.) and the multitude of primary metabolites present. 2 11/30/2012 Natural Product Classification Natural Products can be classified in a variety of ways. Common classifications include structural type and biosynthetic pathways. Common classes include terpenes, steroids, acetogenins, glycosides, and alkaloids. Today’s compounds are classified as a terpene (limonene) and as an acetogenin (eugenol). Terpene Biosynthesis All terpenes are derived biosynthetically from the five-carbon precursor isopentenyl diphosphate. Terpenes are classified by the number of fivecarbon multiples they contain. Structures with two isopentenyl precursor units, like limonene, are called monoterpenes. O O PO32- P O O isopentenyl diphosphate limonene 3 11/30/2012 Acetogenin Biosynthesis Acetogenins are natural products derived from a two-carbon precursor, acetyl coenzyme A. Fatty acids and carbohydrates are also derived from this precursor. Eugenol biosynthesis includes carbohydrate and amino acid intermediates in a long, multi-step synthesis. O CH3CSCoA acetyl CoA HO eugenol OCH3 Isolation of Natural Products Extraction – Many small organic molecules can be extracted from plant material (leaves, flowers, roots) by extraction of the solid material. One example is extraction of ground coffee beans by water. Other compounds could be removed by extraction with acetone or dichloromethane. – This procedure generally gives a mixture of compounds requiring significant further purification. 4 11/30/2012 Isolation Procedures Steam Distillation – In this process, materials that are insoluble in water can be distilled from plant material at temperatures that do not lead to decomposition products (either of the natural product or of the plant material). – Organic molecules that are water soluble are left behind – provided that they don’t distill below the boiling point of water. What is Steam Distillation? Steam distillation occurs when water and an immiscible (insoluble) liquid are heated to boiling. The process is different from distillation of two miscible liquids as in simple distillation or fractional distillation. Let’s review a distillation diagram to see the difference. 5 11/30/2012 Figure 1. Vapor-Liquid Composition for an Ideal Mixture of Two Liquids _ _ _ _ Vapor _ _ 100 _ _ _ _ Temperature OC _ _ _ 90 _ _ _ b _ _ _ _ _ _ a Liquid x _ 80 _ I 100% A 0% B I I I I I 40% A 60% B I I I _ _ _ 0% A 100% B Immiscible Liquids However, if two liquids are immiscible, their vapor pressures are not affected by the presence of the other component – there is no dilution factor. Therefore, the total vapor pressure is the sum of the two independent vapor pressures. 6 11/30/2012 v a p o r p r e s s u r e _ Figure 2. Vapor Pressure for Two Immiscible Liquids _ _ _ _ _ atmospheric pressure _ _ _ _ composite _ _ vapor press. _ _ _ _ _ _ _ _ _ _ water _ _ I 80 I high boiling oil I I I 90 Temperature OC I I I I _ _ _ 100 Steam distillation of natural products Many commercial ‘oils’ derived from natural products are obtained by steam distillation. Examples include fragrance oils (rose oil, peppermint oil, etc.). Today we will provide a sample of clove oil (obtained by steam distillation of clove buds, leaves, or stem). The major component of this oil is eugenol, which can be separated from other ingredients by base extraction (recall extraction of benzoic acid). 7 11/30/2012 Steam Distillation of Natural Products The steam distillate of orange rinds consists of greater than 90% limonene. The other 10% contains a small amount of large number of other terpenes, and some long chain aldehydes and alcohols. Limonene is what gives citrus fruits their aroma and it can be used as an non-toxic insecticide. The amount of limonene in orange rind is small, so you will not obtain a lot of material in this experiment. Procedure You will work in pairs today, BUT EACH OF YOU NEEDS A BIN. One student will steam distill orange peel to obtain limonene and the other will extract eugenol from clove oil. Make sure you observe the procedure and results of your partner. NOTE: You will obtain your clove oil sample from a dispenser at the chemical storeroom door. Wear goggles, and take a dry graduated cylinder to contain the sample. You will not be allowed to start this experiment over if you botch the separation! 8 11/30/2012 Procedure (Cont.) Use the coarse side of the grater to obtain your orange peel. (See picture.) Procedure (Cont.) IMPORTANT: Make SURE you know, for your extraction, which layer in the separatory funnel is the organic layer. They are NOT the same for dichloromethane and for ethyl acetate. NOTE: IF YOUR CALCIUM CHLORIDE DISSOLVES, YOU HAVE A WATER LAYER! STOP AND GET THE OTHER LAYER. Calcium chloride will NOT dissolve in ethyl acetate! Dried ethyl acetate WILL NOT be cloudy! 9 11/30/2012 Procedure (Cont.) SOLVENT REMOVAL: In the final step with both natural products, you will be distilling solvent AWAY from the natural product. The products will remain in the distillation pot; the distillate will NOT contain product. The dichloromethane distills at just under 40o, while the ethyl acetate distills at 77o, so use a higher Variac setting for ethyl acetate. Procedure (Cont.) Essentially all solvent must be removed by distillation from eugenol; with limonene you need to remove about half of the solvent. After the products have been isolated, you will examine their chemistry with classification tests. BOTH products will be analyzed by IR, and the limonene will be analyzed by GC also (if the IR spectrum indicates that water is not present in your sample). 10 11/30/2012 Classification Tests Classification tests are chemical reactions that are specific to a limited number of functional groups and give a visual indicator that reaction has occurred. HO limonene OCH3 eugenol Classification Tests - Eugenol Perform ferric chloride, permanganate, and bromine tests on your eugenol product. Also perform the permanganate and bromine tests on the cyclohexene and cyclohexane standards. Take an IR spectrum of your product and compare with the reference spectrum for eugenol. 11 11/30/2012 Classification Tests - Limonene You will conduct permanganate and bromine tests on the ethyl acetate solution of limonene after concentration. Also perform these tests on the cyclohexene and cyclohexane standards. You MUST take an IR spectrum of your limonene. IF your classification tests are positive AND the IR does not show primarily water, you will analyze your limonene by GC. GC of Limonene Your limonene sample for GC needs to be in a sample vial or small beaker, NOT in a round-bottomed flask. 12 11/30/2012 Safety Sodium hydroxide and hydrochloric acid solutions are corrosive. The bromine solution can cause serious skin burns. Wash with copious amounts of water and rinse with dilute sodium thiosulfite solution. Be careful with the grater; we don’t need skinned knuckles or blood and skin chemicals in the limonene. Cleanup Dispose of citrus peel in the waste basket. Aqueous residues and washes can be disposed of in the sink. All methylene chloride and ethyl acetate solutions can be combined and disposed of in the labeled container in the hood. Limonene and eugenol go in a bottle in the hood. Calcium chloride must also be disposed of in the hood. 13