Chapter III
advertisement

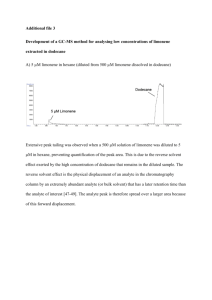
Chapter III Materials and Methodology A. Materials and Equipment Materials Equipment Orange Peelings Direct Steam Distillation Set-up Appliance Styrofoam thermometer Water Thermometer adapter Sidearm adapter Boiling flask Heating mantle Condenser Vacuum adapter Receiving flask Wire or rubber band Triple beam balance Rules, cutter, scissors B. Procedure The conduct of the research will take four phases. These phases include the - a) preparation of samples to be investigated, b) determination of density, durability, and thermal conductivity of the samples through calculations, c) determination of the color and texture through a survey, and lastly d) comparison of the gathered characteristics using inferential statistics. The detailed description of each phase is provided below. Preparation of Samples The researcher will get home appliance Styrofoam and cut six (6) rectangular prisms of dimensions A”xB”XC” out of it. Three of the prisms will be used as the controlled variable (undissolved sample). The other three will be dissolved using limonene as solvent (experimental variable). The limonene will be extracted from the peelings of orange fruits. Extraction shall be done using Direct Steam Distillation (Marmor, 1981). The procedure for such technique is given in the section below. However, as soon as limonene will be extracted from the orange peelings, ___ mL of limonene shall be introduced into three of the of Styrofoam prisms. The prisms shall be allowed to dissolve and solidify again. The combination of the limonene and Styrofoam shall now be the experimental set-up. Isolation of Limonene from Orange Peelings (Direct Steam Distillation) Peel the skin off the oranges and weigh it. The best results are obtained if a fresh Valencia orange is used. Construct an apparatus for "direct steam distillation", as shown below. Place the peel in a blender with about 150 ml of water and blend for about 30 sec. Transfer the slush to the 500 mL distillation flask, using an additional 50 ml of water as a rinse. The flask should be no more than half full or it may boil over during distillation. Heat the mixture to a boil and begin the distillation. Record the temperature after the 1st drop is collected and again after around 20 drops have been collected. At the beginning of the distillation, the mixture may come over cloudy as limonene separates from the water. Continue the distillation until at least 25 ml has been collected and the distillate coming over is clear. The continued presence of limonene in the distillate can best be determined by collecting approximately 1 ml in a test tube and looking for small oil droplets as the test tube is agitated. Transfer the distillate to a separatory funnel. Let the funnel stand for about 5 minutes to allow complete separation of the layers. Carefully drain off and discard the lower, aqueous layer. Transfer the oily organic layer out of the top of the funnel into a small, tared conical vial using a 9" Pasteur pipette and determine the weight of the limonene. Avoid transferring water with the limonene. If water is transferred, decant the limonene into a new tared vial. If water is still present treat with anhydrous magnesium sulfate of sodium sulfate, pipette the limonene to a dry tared vial and weigh again. Calculate the percent recovery of limonene. Obtain an infrared spectrum and either the refractive index or a microboiling point as your lab instructor suggests. Correct the refractive index to the standard temperature of 20 degrees Celcius. Compare the corrected value with the literature value at 200C. Measure the observed optical rotation by weighing limonene into a 10 ml volumetric flask and adding hexane to the mark. After mixing, fill a 1-dm polarimeter cell with your solution, find the observed optical rotation, and calculate an experimental specific rotation. Calculate the experimental specific rotation as a percentage of the value reported in the literature (% optical purity). Determination of Sample Characteristics Density. The density of all the samples shall be determined by obtaining the mass of the sample and dividing it to its volume. The mass shall be obtained using triple beam balance and the volume shall be mathematically calculated using the formula: 𝑉𝑝𝑟𝑖𝑠𝑚 = (𝑙𝑒𝑛𝑔𝑡ℎ)(𝑤𝑖𝑑𝑡ℎ)(ℎ𝑒𝑖𝑔ℎ𝑡) (eq. 1) Upon obtaining the mass and the volume, the researcher will use the density formula to get the density for both the dissolved and undissolved samples. The said formula is given below: 𝐷𝑒𝑛𝑠𝑖𝑡𝑦 = 𝑚𝑎𝑠𝑠 𝑣𝑜𝑙𝑢𝑚𝑒 (eq. 2) Durability. The durability of the samples will be determined base on the amount of weight the samples could carry before it breaks. The samples will be elevated using planks on both sides. Increasing weights shall be placed in the middle part of the sample where no support is placed. The researchers will record their observations during a particular weight is being placed in a table provided below. Weight Observation 10 N 20 N 30 N 40 N Thermal Conductivity. In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction. Heat transfer across materials of high thermal conductivity occurs at a faster rate than across materials of low thermal conductivity. Correspondingly materials of high thermal conductivity are widely used in heat sink applications and materials of low thermal conductivity are used as thermal insulation. A direct measurement may be made by taping a thermometer to the samples and then submerging the item partially in hot water. The recommended tape to use is electrical or masking or something similar. Every few seconds, record the temperature and the time. Make a graph of temperature versus time for every item, and use the results to make a visual comparison on how quickly each one became hot (www.ehow.com). The researcher will record the change in the sample’s temperature per time interval. Now, using the data gathered from the experiment, the researchers will now use the formula for heat conduction to derive the value of the samples thermal conductivity, k. The formula is given below. 𝑄 𝑡 = 𝑘𝐴(𝑇ℎ𝑜𝑡 − 𝑇𝑐𝑜𝑙𝑑 ) 𝑑 (eq. 3) Color and Texture. The color and texture of the samples shall be determined using survey method. The researchers will gather 30 respondents; all are IDS students, through convenience sampling. The researchers will present the dissolved and undissolved samples before them. The respondents will then rate the samples base on its color and texture using a scale. The results shall then be the basis of the representative color and texture of the samples. Comparison of Characteristics Comparison of characteristics shall be done using tools used in inferential statistics. Both parametric and nonparametric tests are to be used in the comparison of the characteristics of the samples. Parametric comparison of values shall be done for the density, durability, and thermal conductivity of the samples. Non-parametric test shall be used, on the other hand, in comparing the color and texture of both dissolved and undissolved samples. The two inferential tools that will be used in this research are: T – test. The t-test (or student's t-test) gives an indication of the separateness of two sets of measurements, and is thus used to check whether two sets of measures are essentially different (and usually that an experimental effect has been demonstrated). In the context of the research, this text shall determine if there exist significant differences on the density, durability, and thermal conductivity on Styrofoam without limonene and that with limonene. Mann – Whitney U test. The test is a non-parametric statistical hypothesis test for assessing whether two independent samples of observations have equally large values. It is one of the most well-known non-parametric significance tests. In the context of the research, this test shall determine if both samples will exhibit significant differences in terms of color and texture as evaluated by the respondents. All of the calculations that will be done in the research shall be carried through a software called MegaStat Excel, an add-on software to Microsoft Excel that processes statistical calculations.