FFR Steam Distillation of Cloves
advertisement

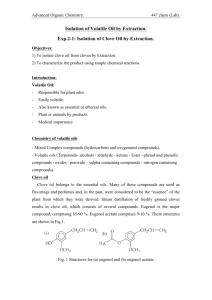
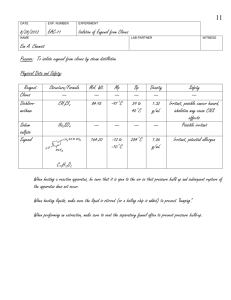
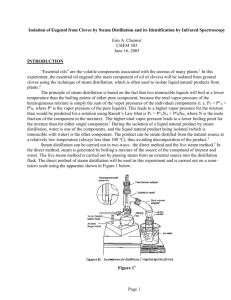
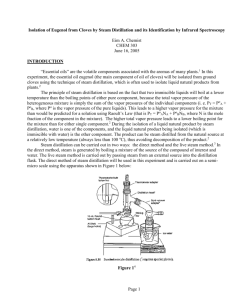
Zach Wright, Chemistry 213 Synthetic #1 FFR Steam Distillation of Clove Buds Introduction Steam Distillation is widely used for separating compounds that are sensitive to temperature that would otherwise be degraded at high temperatures.1 The essential oil found in plants can be co-distilled with water resulting in a non-degraded product. The essential oils derived from many plants have been used for thousands of years by humans to treat for a variety of afflictions.2 During this experiment the essential oil from cloves was extricated from clove buds using steam distillation and the resulting oil was tested for antimicrobial activity. Clove oil extracted from clove buds contains eugenol, caryophyllene, and eugenyl acetate.3 The main component eugenol exhibits some very unique properties including the ability to relieve pain, promote healing. In addition to topical pain relief applications eugenol exhibits antimicrobial properties.4 Eugenyl acetate can be used as a flavoring agent as well as caryophyllene.5 Natural products are important for understanding exactly why certain compounds exhibit extraordinary functions like the essential oils found in cloves. The structure of these naturally occurring compounds can be used as models for other synthetically derived pharmaceutical drugs as well as precursors for other drugs. The understanding of how components interact with the outside world can be accelerated through the study of naturally occurring products. In order to separate the three main temperature sensitive chemical components from the clove buds steam distillation was utilized. Steam distillation takes advantage of the unique properties of water and partial pressure. Water boils at about 100C at atmospheric pressure which is low enough as to not degrade the compounds of the essential oil. The essential oil is immiscible with water and will therefore form a partial pressure that is independent of all other components in the system. The additional pressure from the immiscible clove oil allows water to reach atmospheric pressure faster while being heated and will therefore boil at a temperature slightly below 100C. As water evaporates it van act as a medium of Wright 2 transportation for the volatile vapors of the components that have not fully reached the pressure needed to completely vaporize. Sweeping of the volatile compounds with water as a medium is a slow process and therefore the solution must be carried out over a long period of time. By using stream distillation one can isolated the essential oils from cloves though degradation which allows for a simple extraction for further analysis and identification. The purpose of this experiment was to isolate the essential oils contained in clove buds and to analyze their antimicrobial activity. Isolation was performed by steam distillation and the product composition was analyzed using GC and GC-MS. Experimental Cloves (4 g) were ground to a fine powder and suspended in water (50mL). The immiscible solution was distilled for 2 hours at 100C. The collection flask was kept on ice. The aqueous layer was extracted using dichloromethane (3x15mL). The organic layer was dried over anhydrous sodium sulfate. The solvent was evaporated resulting in a small volume of yellow liquid. (0.366, 6%) GC (phenyl methyl silicone, 40°C to 250°C at 10°C per min) RT 11.62 min, 12.44 min, 13.56 min; GC-MS (phenyl methyl silicone, 40°C to 250°C at 10°C per min) RT 13.43 min, m/z 164.02, 14.06 min, m/z 204.13, 14.85 min, m/z 206.1. Results Discussion and Conclusion The essential oil from cloves was isolated from clove buds using steam distillation to prevent degradation of the oil. After the isolation the cloves were analyzed using a bioassay. Although the percent recovery for this experiment was only 6%, which is not unexpected and correlated well to the reported 5% found in literature, the oil still showed antimicrobial properties. After preparing a culture from the [name of bacteria] two disks were sterilized using acetone. One of the disks was dipped into a solution of the clove oil in acetone and the other was left as it was. Wright 3 The disk was left for an incubation period of 120 hours. From figure one there is a clear retardation of the growth of the bacteria that surrounds the disk that was dipped in the oil from the clove isolation. In contrast bacterial growth can be seen surrounding the white control disks in the bottom left as well as through the rest of the plate. The retardation of bacterial growth surrounding the clove oil disk clearly illustrates the antimicrobial properties of the oil and confirms the numerous reports found in literature. Figure 1 The antimicrobial properties comes from the main component of the oil eugenol. In order to confirm that the oil extracted from the cloves contained eugenol and the two other main components caryophyllene, and eugenyl acetate GC and GC-MS were run on the sample. From the GC(add figure number) and GC-MS(add figure numbers) the clove oil was found to be composed of eugenol 86%, caryophyllene 7% and eugenyl acetate 7%. Although the GC-MS retention times did not match up Wright 4 perfectly to the GC retention times I was able to determine what retention times matched up, based on the order that the compounds should move through the column, with the lightest eugenol having the lowest retention time and eugenyl acetate having the highest retention time. Also the time between the sample retention times was about the same. Table 1 summarizes the % composition of each component determined by GC and GC-MS. Component RT Area m/z % Composition Eugenol 11.62 1.072 164 86 β Caryophyllene 12.44 0.092 204 7 Eugenyl Acetate 13.56 0.086 206 7 Table 1 The essential oil from cloves was isolated from clove buds using steam distillation. The mixture of compounds in the oil was analyzed using GC and GC-MS. The bioassay supported the claims that the main component of clove oil, eugenol, has antimicrobial properties. Add how these results were confirmed. Wright 5