Isomorphous replacement (MIR), cont'd 02-08
advertisement

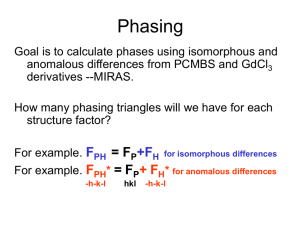
Isomorphous replacement (MIR), cont’d 02-08-06 Lecture 8 Effect of heavy atoms on X-ray intensities • How much difference should we expect by the attachment of one or a few heavy atoms? • Crick and Magdoff (1956) estimated that: – For centric reflections – For acentric reflections And we know that I is proportional to ∑fi2 Effect of heavy atoms on X-ray intensities Attachment of heavy atom causes changes in intensities Native lysozyme A Hg derivative There is no change in reciprocal lattice spacing Another example Native phosphorylase A Hg derivative The problem of non-isomorphism • Ideally, the attachment of heavy atoms only substitutes disordered solvent molecules without causing any structural changes to the crystallized molecule. • Non-isomorphism can be detected by – Changes in unit cell dimensions • Should be <0.5% along each cell axis – Changes in intensities • Orientational changes or conformational changes • Ideal case: 10-20% difference throughout different resolution shells The problem of non-isomorphism – an example Changes in molecule orientation result in changes in diffraction intensities Determination of heavy atom positions • Isomorphous difference Patterson map will give the Patterson function for the heavy atom structure on half the scale. – See the next slide for more information • Heavy atom positions can be solved from the isomorphous different Patterson map. – See the slide after next for a simple example Isomorphous difference Patterson • Patterson function • Isomorphous difference Patterson function where Δ|F|iso = |FPH| - |FP| Isomorphous difference Patterson can be used to solve heavy atom structure - a proof Therefore, An example: how to determine heavy atom positions a (-x2,y2) (x2,y2) (-x1,y1) (x ,y ) 1 1 y1-y2 2x1 2x2 b A 2-fold axis along b axis Space group symmetry: Pm Two atoms in each asymmetric unit We will have four peaks in each asymmetric unit (shown in green) (2x1, 0) (2x2, 0) (x1-x2, y1-y2) (x2-x1, y2-y1) Use the Patterson map to solve heavy atom positions Patterson map symmetry: P2/m The origin along y axis is arbitrary in this Pm space group Now FH can be calculated after the structure of the heavy atoms are determined. FH is the structure factor for the heavy atom part of the derivative. And we have: FPH = FP + FH Single isomorphous replacement • For a centrosymmetric structure, phase angles are either π or 0 – Phases can be determined for one heavy atom derivative Assuming FP and FPH lie in the same direction: (a) If FPH> FP, then FP has the same sign as FH (b) If FPH< FP, then FP has the opposite sign as FH Only rarely does FPH and FP have opposite signs, resulting in: (c) FP has the opposite sign as FH Single isomorphous replacement • For non-centrosymmetric structure – Two phases are possible FP + FH = FPH (1) Draw a circle using radius FP (2) Draw a vector FH with the arrowhead ending on origin (3) Use the starting point of FH as origin, draw a circle using FPH as radius (4) The two circles intercept at two points G and H, giving two phase angles Multiple isomorphous replacement • In theory, phases can be unambiguously determined if there are at least two heavy atom derivatives. – Circle FP and FPH1 intercept at G and H – Circle FP and FPH2 intercept at H and L – Therefore, the correct phase is given by point H • In practice, measurement errors and non-isomorphism result in phase ambiguity and lack of closure error. Lack of closure error If the FH + FP is not equal to FPH, then ε is called the lack of closure error The lack of closure error can be caused by inaccuracy in data collection and nonisomorphism During heavy atom refinement, our goal is to minimize the lack of closure error by adjusting heavy atom’s atomic coordinates, occupany, and temperature factor Protein phase angles To consider the lack of close error, a Gaussian probability distribution is assumed for ε, and for each reflection of each derivative: α varies from 0 to 2π N is the normalization factor E is the root mean square of ε If there are n derivatives, then Phase probability curve First example Reflection j One derivative only Reflection j Two derivatives If there are n derivatives, then Reflection k The most probable phase and the best phase αbest αprobable αprobable αbest The most probable phase is the phase with the highest probability (this does not consider the shape of phase probability curve) The best phase, αbest, is used in calculating electron density maps The best phase and figure of merit m is called the figure of merit Figure of merit is a weighting factor The value of m varies between 0 and 1. Phases with large m values are more accurate. Electron density calculation Figure of merit m is like a weighting factor, the more accurate the phases are, the larger the values of m are. Electron density map Protein coordinates are modeled based on sequence and stereochemistry information