The Effect of Temperature on Solubility of KNO3
advertisement
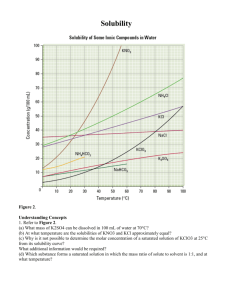
SOLUTION CHEMISTRY Crystal Clear Science! IPC: Science Course Module—Goes to the Movies! University of Houston The Effect of Temperature on Solubility of KNO3 Type of Lesson: Content with Process: Focus on constructing knowledge through active learning. IPC Content TEKS: 9D Learning Goal/ Instructional Goal: Demonstrate how various factors influence solubility including Temperature and nature of the solute and solvent. In this lab students study the effect of temperature on the solubility of KNO3 . Instructional Objectives: • Given different temperatures students are able to explain the effect of increased temperature on the solubility of KNO3. • Given a solubility curve students are able to determine the solubility of various solutes at a given temperature. Key Question: How does temperature affect the solubility of KNO3? Related Process TEKS: (1) Scientific processes. The student, for at least 40% of instructional time, conducts field and laboratory investigations using safe, environmentally appropriate, and ethical practices The student is expected to: (A) demonstrate safe practices during field and laboratory investigations; and (2) Scientific processes. The student uses scientific methods during field and laboratory investigations. The student is expected to: (A) plan and implement experimental procedures including asking questions, formulating testable hypotheses, and selecting equipment and technology; (B) make wise choices in the use and conservation of resources and the disposal or recycling of materials. (B) collect data and make measurements with precision; (C) organize, analyze, evaluate, make inferences, and predict trends from data; and (D) Communicate valid conclusions. (3) Scientific processes. The student uses critical thinking and scientific problem solving to make informed decisions. The student is expected to: (A) analyze, review, and critique scientific explanations, including hypotheses and theories, as to their strengths and weaknesses using scientific evidence and information; (B) draw inferences based on data related to promotional materials for products and services; (C) evaluate the impact of research on scientific thought, society, and the environment; (D) describe connections between physics and chemistry and future careers; and (E) Research and describe the history of physics, chemistry, and contributions of scientists. Science Course Module: Integrated Physics and Chemistry (IPC) 2005 SOLUTION CHEMISTRY To the Teacher: Crystal Clear Science! For all solids there is a limiting amount that will dissolve in a given amount of solvent. Some solids are very soluble in water, while others are nearly completely insoluble. About 200 grams of ordinary sugar will dissolve in 100 grams of water at 25°C, but only about 2 x 10-4 g AgCl will dissolve under those conditions. . Solubility is defined to be the maximum amount of solute that will dissolve in a given amount of solvent at a specified temperature. The solubility of a solid in a given solvent depends on the temperature of the solution. Usually the solubility of a solid increases with increasing temperature, sometimes very noticeably. Typically, solubilities are recorded as grams of solute per 100g of solvent. For many solutes, increasing the temperature increases the solubility, that is, more of the solute dissolves in the same amount of solvent. In this experiment, you will distinguish the temperature behavior of a solution made up of KNO3 dissolved in water. A graph which plots the solubility as a function of temperature is called the solubility curve of the substance. Given such a graph, the solubility of the solid at any temperature may be determined. In this lab students study the effect of temperature on solubility. They are given weighed samples of a soluble salt such as potassium nitrate. The sample is added to 20 ml of water and heated until all of the salt dissolves. The solution is cooled, and the temperature at which solid appears is noted. A solubility curve is prepared by pooling the data from all of the students. • • • • The experiment should be done as a class project, with results posted on the board. Assign samples of KNO3 within a range of 8.00 to 35.00 grams in integral values. Students tend to report the temperature at which the first tiny crystals are sighted, and this value is usually low. A more accurate result is obtained by noting the temperature at which about 5% of the original solid has crystallized. Solubility curves may be produced in a similar way for other salts. Consult solubility curves for the ranges which may be assigned. KClO3 and NH4Cl are two salts which are commonly used in this experiment. Some students may require assistance in the preparation of the solubility curve. Make sure that the students are able to interpret the graph, which indicates the increasing solubility of potassium nitrate with increasing temperature. Time Preparation time: 10 minutes Class Time: 40 minutes Multiple Intelligences: LogicalMathematical Intelligence Consists of the ability to detect patterns, reason deductively and think logically. This intelligence is most often associated with scientific and mathematical thinking. Materials: (for 10 students working in pairs) • 5 400-mL beaker • 5 25 x 200 mm test tube • 5 support stand • 5 heating setup (hot plate; iron ring; wire gauze; burner; striker) • 5 25-mL graduated cylinder • 5 single sided buret clamp • 5 thermometer • 5 35- to 45-cm length heavy gauge copper wire (for stirrer) • 100 g KNO3 • 100 mL distilled water • 1 centigram balance, weighing paper SAFETY NOTE: • • • • • • • These materials may be disposed of in the sink using large amounts of water. Burns when handling hot objects are possible. The potassium nitrate and potassium chlorate are strong oxidizing agents. Handle hot equipment and solutions cautiously and handle the thermometer bulb gently. Wear goggles and aprons. Wash hands thoroughly at the end of the experiment. See Texas Science Safety Manual for lab and investigation guidelines: http://www.tenet.edu/teks/science/safety/safety_manual.html Science Course Module: Integrated Physics and Chemistry (IPC) 2005 SOLUTION CHEMISTRY Engagement: Crystal Clear Science! Texas Tea Drinker! Facilitation Questions: 1. As students walk into class sweeten a glass of iced tea with sugar and drink. 2. As the sugar begins to fall to the bottom of the glass ask students to explain what they think is happening. 3. Ask students what could make the sugar dissolve? Write these responses on the board. Explore: 1. 2. 3. 4. 5. 6. 7. 8. 9. Set up a 400-mL beaker on an iron ring and wire gauze. Add about 300 mL of tap water. Weigh out the amount of potassium nitrate, KNO3 , assigned by the instructor. The sample will range from 8.00 to 35.00 grams. Transfer the solid to a large test tube and add 20 mL (20 g) of distilled water. Stir the mixture with a stirring wire to dissolve as much potassium nitrate as possible. Carefully insert a thermometer. The glass bulb on the bottom of a thermometer is thin and easily broken. Heat the water in the beaker with the Bunsen burner. Clamp the test tube in the water. Use a stirring wire to stir the mixture gently until ALL of the potassium nitrate has dissolved. The temperature may vary from 25 °C to 85 °C depending on the amount of potassium nitrate in the sample. When all of the potassium nitrate has dissolved, loosen the clamp from the support stand and raise the tube out of the water bath. Turn off the burner. Reclamp the tube above and to one side of the beaker. Using the stirring wire, stir the solution gently and observe it as it cools. As soon as crystallization begins, note the temperature of the solution. It is easiest to see crystals by looking at the bottom of the test tube where they will collect as soon as they begin to form. After the crystallization temperature has been recorded, put the test tube back into the water bath and warm the solution until all of the crystals have redissolved. Repeat the cooling procedure to check the crystallization temperature. The two readings should be within 1 degree of each other. If the two readings do not agree within 1 degree, rewarm the solution in the water bath, cool it, and continue until satisfactory agreement is obtained. Your sample mass will be recorded on the blackboard; record the experimental temperature next to it. When the data from all of the student samples are recorded at the board, copy them to draw (graph) a solubility curve for grams of potassium nitrate dissolved per 100 grams of water. Explain: 1. Your data furnish the amount of KNO3 that will be dissolved in 20 g of water at each temperature. For each data point, calculate the amount of KNO3 that would dissolve in 100 g of water. Your calculations will be the solubility of KNO3 in water at each of the temperatures reported. Mass of KNO3 8.00 10.0 15.0 20.0 25.0 30.0 35.0 2. Saturation Temperature °C 26 33 44 57 65 73 83 Mass of KNO3/100g of water 40 50 75 100 125 150 175 Construct the solubility curve for KNO3 . Plot solubility in grams per 100 grams of water on the y-axis and temperature on the x-axis. Extrapolate the curve to include solubility from 10 °C to 100°C. Science Course Module: Integrated Physics and Chemistry (IPC) 2005 SOLUTION CHEMISTRY 3. Crystal Clear Science! Explain how temperature affects the solubility of KNO3. There is a positive correlation between temperature and solubility of KNO3 Elaborate: • • • • Ask students how they would go about testing the solubility of KNO3 in various solvents. The dissolved oxygen content of water is very critical to marine life. Using the internet investigate the solubility of oxygen in water as a function of temperature., Find solubility graphs of other chemicals Discuss how crystal growers use solubility graphs to figure out the following: 1. Which solutes will grow the biggest crystals by cooling? 2. How much solute will be needed to grow the crystal? 3. What temperature must the solution be heated and cooled for the best results? 4. Why do certain solutes such as salt grow such small crystals? Science Course Module: Integrated Physics and Chemistry (IPC) 2005 SOLUTION CHEMISTRY Crystal Clear Science! Evaluate: Scientific Accuracy POINTS Reasoning Communication Collaboration I analyzed the data accurately and answered analysis questions accurately. I communicated answers to the investigation questions completely and thoroughly using correct grammar. I shared my ideas about the investigation in the whole group discussion and with my teammates. I communicated answers to the investigation questions and using correct grammar. I shared some of my ideas about the investigation in the whole group discussion and with my teammates. I worked very well with my group. Each person had a lot of input and participated in the investigation. I analyzed the data with some errors and answered analysis questions with some errors. I communicated answers to the investigation questions and with grammatical errors. I shared a few of my ideas about the investigation in the whole group discussion and with my teammates. I worked somewhat well with my group. Each person had some input and participated in the investigation. I cannot explain how temperature affects solubility of different solutes. I cannot create a solubility curve and read a solubility curve. I analyzed the data incorrectly and answered analysis questions incorrectly. I communicated answers to the investigation questions and with many grammatical errors. I did not share my ideas about the investigation in the whole group discussion and with my teammates. I did not work well with my group. A few people had input and participated in the investigation. Subtotal: ____ Subtotal: ____ Subtotal: ____ I can accurately explain how temperature affects solubility of different solutes. I can accurately create a solubility curve and read a solubility curve. 4 I can explain how temperature affects solubility of different solutes. I can create a solubility curve and read a solubility curve. 3 I can sometimes explain how temperature affects solubility of different solutes. I can sometimes create a solubility curve and read a solubility curve. 2 1 I analyzed the data somewhat accurately and answered analysis questions somewhat accurately. I worked well with my group. Each person had input and participated in the investigation. Subtotal: ____ TAKS-like Items: 1. A crystal of solute is dropped into a solution and it does not dissolve, but falls to the bottom of the flask. Identify the type of solution. A. B. C. D. Dilute Saturated Unsaturated Supersaturated Science Course Module: Integrated Physics and Chemistry (IPC) 2005 TOTAL: ____/16pts SOLUTION CHEMISTRY 2. Crystal Clear Science! Select the statement that lists the various factors that affect the solubility of a solute. A. B. C. D. The The The The solubility solubility solubility solubility of of of of a solute increases by heating the solvent, by adding more solute, and by stirring the solute. a solute increases by cooling the solvent and stirring the solute increases by adding additional solute and stirring. the solute increases by stirring the solute, by heating the solvent, and by crushing the solute. Examine the graph of the solubility curve below to answer question #3. 3. Based on the graph above, approximately how many grams of KNO3 are dissolved in 20 mL of H2O at 60 °C? A. B. C. D. 40 30 10 20 g g g g References/Resources/Websites: • • • Alternate solubility lab: http://www.sciencebyjones.com/solubility_of_unknown_salt.htm Power Point Presentation on solubility http://personal.tcu.edu/~thanna/1122/lecture13/ Animation dissolving rate of NaCl http://www.mpcfaculty.net/mark_bishop/NaCl_dissolves.htm Science Course Module: Integrated Physics and Chemistry (IPC) 2005