Solubility Practice a Curvy Subject
advertisement

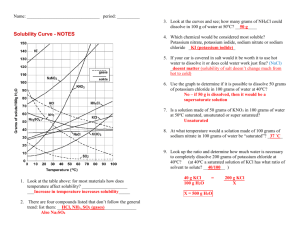
Teacher page Enduring Understanding Resource use is determined by properties and availability. Chemistry I EQ: Why do some substances dissolve in water while others do not? Broad Brush Knowledge Concentrations, Measurement, Colligative Properties, Saturation Solubility Practice a Curvy Subject Concepts Important to Know and Understand Chemical Quantities, Interactions of Matter Core Objectives: Describe the nature of water and aqueous solutions: Describe the structure of the water molecule and explain how its polarity affects its properties such as surface tension, solubility, boiling point, freezing point, and specific heat. Describe solutions in terms of solute, solvent, solvation, degrees of saturation, relative concentration, and colligative properties. TEKS 12A demonstrate and explain effects of temperature and the nature of solid solutes on the solubility of solids. 13 The student knows relationships among the concentration, electrical conductivity, and colligative properties of a solution. The student is expected to: A: compare unsaturated, saturated, and supersaturated solutions B: interpret relationships among ionic and covalent compounds, electrical conductivity, and colligative properties of water, 2D: organize, analyze, evaluate, make inferences, and predict trends from data; 2E: communicate valid conclusions. TEACHER MANAGEMENT Estimated Time: 20-30 minutes could be used as homework in Unit I Section C.1. Materials: Copies for all students Teacher Prep: None Safety Precautions: None PRACTICE PURPOSE: to practice reading and interpreting solubility graphs. Solubility Graph of Solids and Gases KI NaNO3 KNO3 140 130 120 Grams of solute / 100. g H2O 110 100 90 NH4Cl 80 70 KClO3 60 KCl 50 HCl NaCl 40 30 20 10 NH3 SO2 0 10 20 30 40 50 60 70 80 90 100 Teacher Page Temperature (0C) KEY Directions: Answer in complete sentences and show all work for calculations. 1. What relationship exists between solubility and temperature for most of the substances shown? A direct relationship is shown for most substances, as temperature increases the solubility of most substances increases. 2. What is the exception? The exception is gas solubility. Gases are less soluble at higher temperature, illustrating an indirect relationship 3. What explains why solids become more soluble as temperature increases and why gases become less soluble? Use the following words to help justify your response: temperature, collisions and energy. As temperature increases both speed of the water molecules and the number of collisions increase. A solid remains dissolved by the continual motion of the eater molecules. The more energy and motion, the more particles that can remain in solution. This is especially true for ionic compounds, because water is polar and the opposite charges help stick the water molecules to the ions. Gases are often nonpolar and they have very low boiling points, so as temperature increases they tend to “boil away”. That is why a soda will go flat much faster if it is left in a warm place. 4. Which is more soluble NaNO3 or KCl? Does the temperature matter? Why or why not? NaNO3 because the higher line indicates that more NaNO3 can be dissolved at ALL temperatures. 5. How does the line drawn for a particular substance relate to the saturation of a solution of that substance? Use the following words to help justify your response: saturated, unsaturated, supersaturated. The line marks the saturation point of a solute in 100 grams of water. If you have less grams dissolved than indicated by the line then your solution is unsaturated at that temperature. If you have more, then your solution is supersaturated at that temperature. 6. How many grams of NH4Cl will dissolve in 100. grams of water at 90.0C? 72 grams 7. How many grams of KClO3 will dissolve in 300. grams of water at 30.0C? 300. g of H2O X 10. g KClO3 = 30. g KClO3 100. g H2O 8. How would you make a saturated solution of KNO3 at 60.0C in 50. grams of water? The graph indicates that that 106 grams of KNO3 will dissolve in 100. grams of water, so I need only 106g /2= 53 grams of KNO3 to make the solution. So I would measure out 50 grams of water and 53 grams of KNO3 and mix them together to make the solution. 9. If you were asked to make a saturated solution of KCl in 100. grams of water, what other piece of information would you need to before you could start? Why? You would need to know the temperature of the solution. The saturation point is very different depending on the temperature 10. If you start with a saturated solution of NH3 in 100. grams of water at 10.0C, how many grams of NH3 gas would bubble out of solution if you raise the temperature to 80.0C? Teacher Page 100. grams of water at 10.0C can hold 70. grams of NH3. 100. grams of water at 80.0C can hold 14 grams of NH3. So 70. g -14 g = 56 grams of NH3 will bubble out. 11. A saturated solution of NaNO3 was made with 300. grams of water at 40.0C. How much NaNO3 could be recovered by evaporating the solution to dryness? So 300. grams of water can hold 3 X 106 g = 318 g NaNO3 will be recovered. 12. A saturated solution KNO3 in 400. grams of water at 50.0C is cooled to 10.0C. How much KNO3 will come out of solution as crystals? From the graph 100. grams of water at 50.0C can hold 83 g of KNO3 So 400. grams of water can hold 4 X 83 g = 332 g KNO3 From the graph 100. grams of water at 10.0C can hold 21 g of KNO3 So 400. grams of water can hold 4 X 21 g = 84 g KNO3 As the solution is cooled 332 g- 84g = 248 g KNO3 will form crystals. 13. You start with 200. grams of ice saturated with SO2 at 0.0C. How many grams of SO2 will bubble out of solution if you melt the ice and raise the temperature of the water to 80.0C? From the graph 100.g of ice at 0.0C can hold 23 g of SO2 So 200. grams of ice can hold 2 X 24 g = 48 g SO2 From the graph 100g. of water at 80.0C can hold 4 g of SO2 So 200. grams of water can hold 2 X 4g = 8 g of SO2 As the solution is heated 48 g – 8 g = 40 g of SO2 will bubble out.