Micrococcaceae Biochemical Tests and Media
advertisement
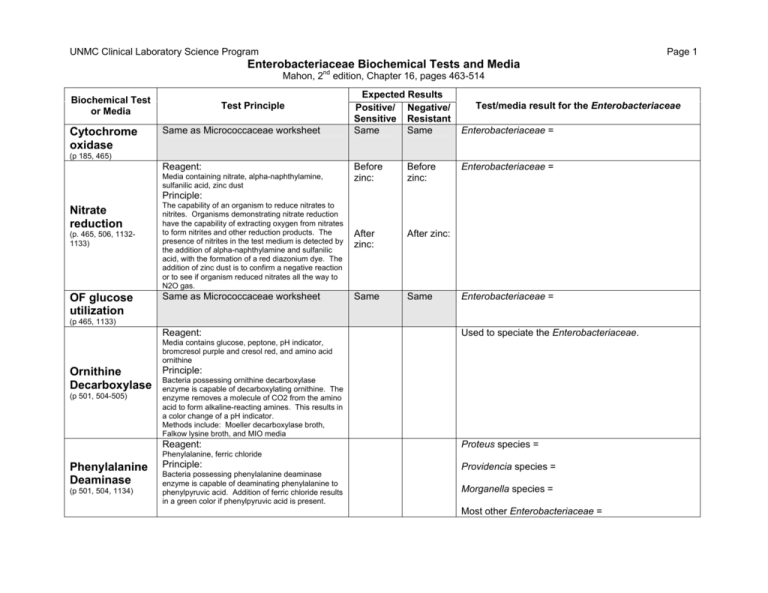
UNMC Clinical Laboratory Science Program Page 1 Enterobacteriaceae Biochemical Tests and Media Mahon, 2nd edition, Chapter 16, pages 463-514 Biochemical Test or Media Cytochrome oxidase Test Principle Same as Micrococcaceae worksheet Expected Results Positive/ Negative/ Sensitive Resistant Same Same Test/media result for the Enterobacteriaceae Enterobacteriaceae = (p 185, 465) Reagent: Media containing nitrate, alpha-naphthylamine, sulfanilic acid, zinc dust Before zinc: Before zinc: After zinc: After zinc: Same Same Enterobacteriaceae = Principle: Nitrate reduction (p. 465, 506, 11321133) OF glucose utilization The capability of an organism to reduce nitrates to nitrites. Organisms demonstrating nitrate reduction have the capability of extracting oxygen from nitrates to form nitrites and other reduction products. The presence of nitrites in the test medium is detected by the addition of alpha-naphthylamine and sulfanilic acid, with the formation of a red diazonium dye. The addition of zinc dust is to confirm a negative reaction or to see if organism reduced nitrates all the way to N2O gas. Same as Micrococcaceae worksheet Enterobacteriaceae = (p 465, 1133) Reagent: Used to speciate the Enterobacteriaceae. Media contains glucose, peptone, pH indicator, bromcresol purple and cresol red, and amino acid ornithine Principle: Ornithine Bacteria possessing ornithine decarboxylase Decarboxylase enzyme is capable of decarboxylating ornithine. (p 501, 504-505) The enzyme removes a molecule of CO2 from the amino acid to form alkaline-reacting amines. This results in a color change of a pH indicator. Methods include: Moeller decarboxylase broth, Falkow lysine broth, and MIO media Reagent: Proteus species = Phenylalanine, ferric chloride Phenylalanine Deaminase (p 501, 504, 1134) Principle: Bacteria possessing phenylalanine deaminase enzyme is capable of deaminating phenylalanine to phenylpyruvic acid. Addition of ferric chloride results in a green color if phenylpyruvic acid is present. Providencia species = Morganella species = Most other Enterobacteriaceae = UNMC Clinical Laboratory Science Program Page 2 Enterobacteriaceae Biochemical Tests and Media Mahon, 2nd edition, Chapter 16, pages 463-514 Biochemical Test or Media Indole production (p 185, 467, 501-502, 505) Citrate utilization (p 467, 501-502, 1122) Gelatin liquefaction (p: 492-495, 1126) Test Principle Used to speciate the Enterobacteriaceae. Principle: Escherichia coli = Bacteria that possess the enzyme tryptophanase are capable of cleaving tryptophan, producing indole, pyruvic acid and ammonia. Indole can be detected by adding para-dimethylaminobenzaldehyde (Ehrlich’s or Kovac’s reagent) and observing a red color. Reagent: Sodium citrate, pH indicator This test is determines the ability of an organism to utilize sodium citrate as its only carbon source for metabolism. Bacteria that can grow on this medium turn the bromthymol blue indicator from green to blue. Reagent: Gelatin Escherichia coli = Klebsiella species = Proteus species = Principle: This test is used to determine the ability of an organism to produce proteolytic enzymes (gelatinases) that liquefy gelatin. Motility Principle: (p 465, 501, 505, 11311132) Used to determine if an organism is motile. An organism must possess flagella to be motile. If organism is motile, it will spread out into the medium from the site of inoculation. Reagent: o-nitrophenyl-β-D-galactopyranoside (ONPG) Principle: (p 185, 498-499) Used to speciate the Enterobacteriaceae. Principle: <0.4% agar concentration in media to allow free spread of organism (o-nitrophenyl galactosidase) Test/media result for the Enterobacteriaceae Reagent: Amino acid tryptophan in broth, para-dimethylaminobenzaldehyde Reagent: ONPG test Expected Results Positive/ Negative/ Sensitive Resistant This test is used to determine the ability of an organism to produce β -galactosidase, an enzyme that hydrolyzes the substrate ONPG to form a visible (yellow) product, orthonitrophenol. This determines lactose fermentation in slow lactose fermenters. This test is not a substitute for the determination of lactose fermentation because only the enzyme β-galactosidase is measured. Serratia species = Most other Enterobacteriaceae = Klebsiella = Shigella = Yersinia = Most other Enterobacteriaceae = Used to speciate the Enterobacteriaceae. UNMC Clinical Laboratory Science Program Page 3 Enterobacteriaceae Biochemical Tests and Media Mahon, 2nd edition, Chapter 16, pages 463-514 Biochemical Test or Media VogesProskauer (Acetylmethylcarbinol) (p 198, 467, 498, 500501, 1131) Test Principle Reagent: Principle: Expected Results Positive/ Negative/ Sensitive Resistant Test/media result for the Enterobacteriaceae Used to speciate the Enterobacteriaceae. Potassium hydroxide, alpha-naphthol This test is used to determine the ability of an organism to metabolize glucose to pyruvic acid, then to acetyl-methyl-carbinol (acetoin). In the presence of atmospheric oxygen and 40% KOH, acetoin is converted to diacetyl, and then alphanaphthol is added to catalyze or bring out a red complex. Reagent: Urea Used to speciate the Enterobacteriaceae. Principle: Urease production (p 185, 467, 501, 503, 1139) Urease is an enzyme possessed by many species of microorganisms that can hydrolyze urea to ammonia, CO2 and H2O. The ammonia reacts in solution to form ammonium carbonate, resulting in alkalinization and an increase in the pH of the medium. The pH change will cause the phenol red indicator to change to red. Ingredients: A/A = Medium in tube as a slant contains agar base, dextrose, lactose (10x concentration of dextrose), phenol red and ferrous sulfate. Medium is red uninoculated. K/K = Examples of Enterobacteriaceae that are lactose fermenters: Principle: KIA (p. 497-499, 11271128) This test is used to determine the ability of an organism to ferment glucose and lactose and produce hydrogen sulfide. Fermentation of glucose but not lactose, changes the color from red to yellow (butt and slant) initially. With continued incubation, slant changes back to red due to reversion of reaction under aerobic conditions. Butt remains yellow due to anaerobic conditions. Fermentation of both glucose and lactose changes color from red to yellow, and both butt and slant stay yellow due to high levels of lactose being fermented. Black precipitate forms when organism is able to produce H2S from ferrous sulfate. K/A = Examples of Enterobacteriaceae that are non-lactose fermenters: Black precipitate = Gas = UNMC Clinical Laboratory Science Program Page 4 Enterobacteriaceae Biochemical Tests and Media Mahon, 2nd edition, Chapter 16, pages 463-514 Ingredients: Expected Results Positive/ Negative/ Sensitive Resistant A/A = Principle: K/K = Biochemical Test or Media Test Principle Medium in tube as a slant contains agar base, dextrose, lactose and sucrose (both 10x concentration of dextrose), phenol red and ferrous sulfate. Medium is red uninoculated. TSI (p 497-499, 1138) This test is used to determine the ability of an organism to ferment glucose, lactose, and/or sucrose, and produce hydrogen sulfide. Fermentation of dextrose but no lactose or sucrose, changes the color from red to yellow (butt and slant) initially. With continued incubation, slant changes back to red due to reversion of reaction under aerobic conditions. Butt remains yellow due to anaerobic conditions. Fermentation of dextrose plus lactose and/or sucrose changes color from red to yellow, and both butt and slant stay yellow due to high levels of lactose being fermented. Black precipitate forms when organism is able to produce H2S from ferrous sulfate. Ingredients: Medium in tube as a slant contains agar base, lysine, small amount of glucose, ferric ammonium citrate, sodium thiosulfate and bromcresol purple (pH indicator). Medium is purple uninoculated. LIA (p 505, 1129) Used to speciate the Enterobacteriaceae. K/A = Black precipitate = Gas = LDA = LDA = Organism Proteus species Principle: This test is used to determine whether a GNR decarboxylates or deaminates lysine and forms hydrogen sulfide. Butt: Glucose fermentation (acidic) will turn butt yellow. If the organism produces lysine decarboxylase, cadaverine is formed. Cadaverine neutralizes the organism acids formed by glucose fermentation, and the butt reverts to alkaline state (purple). If decarboxylase is not produced, butt remains acidic (yellow). Slant: If the oxidative deamination of lysine occurs, a compound is formed that, in the presence of ferric ammonium citrate and a coenzyme, flavin mononucleotide, forms a burgundy (wine-red) color on the slant. If deamination does not occur, the LIA slant remains purple. Black precipitate = H2S production from ferric ammonium citrate. Test/media result for the Enterobacteriaceae Providencia species LDC = LDC = Salmonella species Citrobacter species H2S = H2S = LDA LDC H2S UNMC Clinical Laboratory Science Program Page 5 Enterobacteriaceae Biochemical Tests and Media Mahon, 2nd edition, Chapter 16, pages 463-514 Biochemical Test or Media Test Principle Media classification: Selective and differential for GNR Ingredients: MacConkey agar (p 314-316, 490-491, 964, 1129-1130) Peptone base with lactose, gram positive organisms inhibited by crystal violet and bile salts. Neutral red as indicator. Principle: pH indicator changes to dark pink/red when organism ferments lactose. Non-lactose fermenters remain clear and colorless. Media classification: Selective and differential for GNR – esp. Salmonella and Shigella Hektoen enteric agar (HE) (p 490-491, 964, 11261127) Ingredients: Peptone base agar with bile salts, lactose, sucrose, salicin, and ferric ammonium citrate. Indicators include bromthymol blue and acid fuchsin. Expected Results Positive/ Negative/ Sensitive Resistant Test/media result for the Enterobacteriaceae Examples of Enterobacteriaceae that are lactose fermenters: Examples of Enterobacteriaceae that are non-lactose fermenters: Colony morphology: E. coli = Salmonella = Principle: Gram positive organisms and some coliforms are inhibited by high bile salt concentration. GNR that will grow can be differentiated as to lactose/sucrose fermenters / non-fermenters. H2S production can also be determined. Shigella = Media classification: Colony morphology: Differential, selective medium for the isolation and differentiation of Salmonella and Shigella spp. from other gram negative enteric bacilli. E. coli = Ingredients: Xylose lysine desoxycholate agar (XLD) (p 490-491, 964, 11391140) Yeast extract agar with lysine, xylose, lactose, sucrose, and ferric ammonium citrate. Sodium desoxycholate inhibits gram positive organisms; phenol red as indicator. Principle: Sucrose, lactose and xylose can be fermented. Lysine can be decarboxylated. Sodium thiosulfate can be used for production of H2S. Salmonella = Shigella = Citrobacter =








