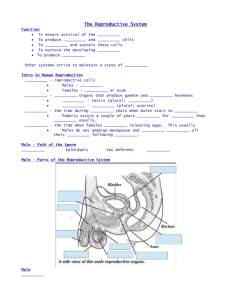
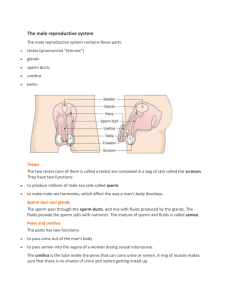
Components of fractionated stallion seminal plasma and the
advertisement

Department of Production Animal Medicine Faculty of Veterinary Medicine University of Helsinki Finland Components of fractionated stallion seminal plasma and the effects of seminal plasma on sperm longevity Maria Kareskoski ACADEMIC DISSERTATION To be presented for public criticism, with permission of the Faculty of Veterinary Medicine, University of Helsinki, in Lecture Room 4, Latokartanonkaari 7 (B-building), Helsinki, on April 8th, 2011, at 12 noon. Supervisors Professor Terttu Katila DVM, MS, PhD, Dipl. ECAR Department of Production Animal Medicine Faculty of Veterinary Medicine University of Helsinki Finland Professor Magnus Andersson DVM, PhD, Dipl. ECAR Department of Production Animal Medicine Faculty of Veterinary Medicine University of Helsinki Finland Professor Heriberto Rodriguez-Martinez DVM, PhD, Dipl. ECAR Department of Clinical and Experimental Medicine Faculty of Health Sciences Linköping University Sweden Reviewers Professor Anne-Marie Dalin DVM, PhD, Dipl. ECAR Department of Clinical Sciences Division of Comparative Reproduction Swedish University of Agricultural Sciences Sweden Senior researcher Michèle Magistrini PhD Joint Research Unit for Reproductive and Behavioural Physiology Institut National de la recherche Agronomique (INRA) France Opponent Professor Harald Sieme Reproduktionsmedizinische Einheit der Kliniken Tierärztliche Hochschule Hannover Germany Chairman Professor Terttu Katila DVM, PhD, Dipl. ECAR Department of Production Animal Medicine Faculty of Veterinary Medicine University of Helsinki Finland ISBN 978-952-92-8738-3 (Paperback) ISBN 978-952-10-6899-7 (PDF) http://ethesis.helsinki.fi Unigrafia Oy Helsinki 2011 2 I dedicate this work to a friend lost too early. The grief of those who lost you The pride of having known you Makes the rain seem colder now Author unknown 3 Contents Abstract................................................................................................................................................... 7 List of original publications ..................................................................................................................... 9 Abbreviations ........................................................................................................................................ 10 1. Introduction ................................................................................................................................... 12 2. Review of the literature...................................................................................................................... 13 2.1. Ejaculation of the stallion and characteristics of stallion semen ................................................... 13 2.2. Anatomy and function of the accessory glands ............................................................................ 14 2.3. Semen collection and semen processing for storage ..................................................................... 15 2.4. The composition of stallion seminal plasma ................................................................................ 16 2.4.1. Enzymes .............................................................................................................................. 16 2.4.2. Other protein compounds ..................................................................................................... 18 2.4.3. Prostaglandins ...................................................................................................................... 20 2.4.4. Electrolytes and trace elements ............................................................................................. 21 2.4.5. Other components studied in stallion seminal plasma ........................................................... 22 2.5. The effects of seminal plasma on sperm during storage ............................................................... 23 2.6. The effects of seminal plasma in the genital tract of the mare ...................................................... 25 2.7. Research objectives ..................................................................................................................... 26 3. Aims ................................................................................................................................................. 27 4. Material and methods ........................................................................................................................ 28 4.1. Animals ...................................................................................................................................... 30 4.1.1. Experiment A (I: Trial 1, II: Trial 1) and Experiment B (I: Trial 2) ....................................... 30 4.1.2. Experiment C (II: Trial 2, III: Trial 1)................................................................................... 30 4.1.3. Experiment D (II: Trial 3, III: Trial 2) .................................................................................. 31 4.1.4. Experiment E (IV) ................................................................................................................ 31 4.2. Semen collection......................................................................................................................... 31 4.2.1. Experiment A (I: Trial, II: Trial 1) ........................................................................................ 32 4.2.2. Experiment B (I: Trial 2) ...................................................................................................... 32 4.2.3. Experiment C (II: Trial 2, III: Trial 1)................................................................................... 32 4.2.4. Experiment D (II: Trial 3, III: Trial 2) .................................................................................. 34 4.2.5. Experiment E (IV) ................................................................................................................ 34 4.3. Semen handling and preparation of samples ................................................................................ 36 4 4.3.1. Experiment A (I: Trial 1, II: Trial 1) ..................................................................................... 36 4.3.2. Experiment B (I: Trial 2) ...................................................................................................... 37 4.3.3. Experiment C (II: Trial 2, III: Trial 1)................................................................................... 37 4.3.4. Experiment D (II: Trial 3, III: Trial 2) .................................................................................. 37 4.3.5. Experiment E (IV) ................................................................................................................ 38 4.4. Sperm quality assessment............................................................................................................ 38 4.4.1 Sperm motility (I, III, IV) ...................................................................................................... 38 4.4.2. Plasma membrane integrity (PMI) (I) ................................................................................... 39 4.4.3. Sperm morphology staining and evaluation (IV) ................................................................... 39 4.4.4. Sperm chromatin structure assay (IV) ................................................................................... 39 4.5. Determination of enzyme and electrolyte levels in seminal plasma (II, IV) .................................. 39 4.6. Protein analyses of seminal plasma (III) ...................................................................................... 40 4.7. Fertility data (IV) ........................................................................................................................ 41 4.8. Statistical analyses ...................................................................................................................... 41 4.8.1. Experiments A-D (I, II, III) .................................................................................................. 41 4.8.2. Experiment E (IV) ................................................................................................................ 42 5. Results .............................................................................................................................................. 43 5.1. Effects of seminal plasma fractions and seminal plasma removal on sperm survival during storage (I, IV) ................................................................................................................................................ 43 5.1.1. Effects of seminal plasma removal on sperm longevity (I, IV) .............................................. 43 5.1.2. Differences between seminal plasma fractions in the effects on sperm survival during storage (I, IV) ............................................................................................................................................ 46 5.1.3. Differences between stallions in sperm survival during storage (I) ........................................ 46 5.1.4. The effects of seminal plasma exchange between stallions on sperm motility (III) ................ 49 5.2. Levels of alkaline phosphatase, acid phosphatase, -glucuronidase and electrolytes in seminal plasma fractions (II, IV) ..................................................................................................................... 50 5.2.1. Differences between seminal plasma fractions (II, IV) .......................................................... 50 5.2.2. Differences between stallions (II) ......................................................................................... 51 5.2.3. Correlations of the levels of enzymes and electrolytes in seminal plasma with semen quality, sperm longevity, and fertility (II, IV) ............................................................................................. 52 5.3. Proteins in seminal plasma fractions and correlations with sperm concentration and motility (III) 54 5.3.1. Differences in protein levels between stallions and seminal plasma fractions (III) ................. 54 5.4. The association between seminal plasma components and semen quality, sperm longevity, and fertility (III, IV) ................................................................................................................................. 56 5 6. Discussion ......................................................................................................................................... 58 6.1. Effects of the removal of seminal plasma on sperm longevity (I, IV) ........................................... 58 6.2. Sperm survival during storage in sperm-rich and sperm-poor fractions (I, IV) ............................. 60 6.3. Levels of seminal plasma components in sperm-rich and sperm-poor fractions (II, III, IV) .......... 61 6.4. Contents of seminal plasma in relation to sperm survival during storage and fertility (III, IV) ...... 62 7. Conclusions ....................................................................................................................................... 65 Acknowledgments ................................................................................................................................. 66 References............................................................................................................................................. 68 Original articles ..................................................................................................................................... 83 6 Abstract Seminal plasma (SP) is the fluid portion of semen, secreted by the epididymides and the accessory glands before and during ejaculation. The functions of SP include acting as a vehicle transporting ejaculated spermatozoa through the urethra, and it also plays a role in the transport, protection and nutrition of spermatozoa in the female genital tract. Seminal factors participate in the regulation in sperm capacitation, the acrosome reaction and gamete interaction. The stallion’s ejaculate is a series of jets that differ in sperm concentration, semen volume and biochemical composition. Before the actual ejaculation, a clear and watery pre-sperm fluid is secreted. The first three jets form the sperm-rich fractions, and contain ¾ of the total number of sperm. The semen volume and sperm concentration in each of the jets decrease towards the end of the ejaculation, and the last jets are sperm-poor fractions with a low sperm concentration. The aims of these studies were to examine the effects of the different SP fractions, and the presence of SP, on sperm survival during storage. Pre-sperm fluid, and semen fractions with a high (sperm-rich) and low (sperm-poor) sperm concentration were collected in five experiments. The levels of selected enzymes, electrolytes and proteins in different SP fractions were determined. These studies also aimed at assessing the individual variation in the levels of the selected SP components and in the effects of SP on spermatozoa. The association between the components of SP and semen quality, sperm longevity, and fertility was examined with a stepwise linear regression analysis. Sperm motility and plasma membrane integrity (PMI) in fractionated ejaculates were evaluated after 24 h of cooled storage (Experiment A) or freezing and thawing (Experiment B), with or without the presence of SP. In Experiment C, four fractionated ejaculates were collected from each of two reproductively normal stallions and two subfertile stallions. Sperm motility was determined after 24h of cooled storage with or without SP, and SP was also exchanged pairwise between normal and subfertile stallions. In experiment D, six fractionated ejaculates were collected from each of seven stallions, and SP was separated from spermatozoa through centrifugation and filtration. The levels of alkaline phosphatase, acid phosphatase, calcium, magnesium, sodium, potassium and inorganic phosphate were determined in SP samples collected in Experiments A, C, D and E. The level of glucuronidase was determined in Experiments A, C, and D, and prostaglandin E2 in Experiment E. The samples in Experiment C were subjected to reverse-phase highperformance liquid chromatography (RP-HPLC), N-terminal amino acid sequence analysis, and mass spectrometry in order to study the protein content in the SP fractions. The protein profiles in the SP fractions collected in Experiment D were analyzed with sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and densitometry. In Experiment E, fractionated ejaculates were collected from 43 stallions (one ejaculate/stallion) at nine stud farms. DNA integrity, measured with the sperm chromatin structure assay, and normal sperm morphology were determined before storage. After 24h 7 of cold storage, sperm motility and DNA integrity were determined in samples stored with or without SP. The first cycle pregnancy rate was calculated for all the reported matings with the studied stallions in the year the samples were collected. Compared to samples containing SP during storage, centrifugation and the subsequent removal of SP reduced sperm motility parameters during 24 h of cooled storage in all SP fractions, but sperm membrane integrity was not affected. Some of the measured post-thaw motility parameters were also higher in samples containing SP compared to samples stored without SP. In contrast, the proportion of DNA-damaged spermatozoa was greater in the samples stored with SP than those without SP, and this effect was seen in both sperm-rich and sperm-poor fractions. There were no differences in DNA integrity between fractions stored with SP, but the sperm-rich fraction showed less DNA damage than the sperm-poor fraction after SP removal. The differences between fractions in sperm motility after cooled storage were non-significant. The levels of alkaline phosphatase, acid phosphatase and -glucuronidase were higher in the sperm-rich fractions compared to the sperm-poor fractions, while the concentrations of calcium and magnesium were higher in sperm-poor fractions than in sperm-rich fractions. The concentrations of sodium and chloride were highest in pre-sperm fluid. Three horse seminal proteins (HSP-1, HSP-2, and HSP-4) were detected in the SP samples of all stallions. HSP-1 was the major protein in all fractions, all stallions, and all ejaculates. No significant differences emerged between fractions or stallions in the number of peaks in liquid chromatography. The levels of HSP-1 and HSP-2 were correlated with the sperm concentration. Gel electrophoresis revealed 6–12 protein bands in stallion SP. The amount of proteins at 60–70 kD was greater in sperm-poor fractions than in sperm-rich fractions and whole ejaculates. In the sperm-poor fraction, the level of potassium was associated with the maintenance of sperm motility during storage. The levels of alkaline and acid phosphatase were associated with sperm concentration and the total number of spermatozoa in the ejaculates. None of the measured SP components were correlated to the first cycle pregnancy rate. In summary, the removal of SP improved DNA integrity after cooled storage compared with samples containing SP. There were no differences in the maintenance of sperm motility between the sperm-rich and sperm-poor fractions and whole ejaculates during cooled storage, irrespective of the presence of SP. The lowest rate of DNA damage was found in the sperm-rich fractions stored without SP. In practice, the results presented in this thesis support the use of individual modifications of semen processing techniques for cooled transported semen from subfertile stallions. 8 List of original publications This thesis is based on the following original publications referred to in the text by their Roman numerals: I Kareskoski AM, Reilas T, Andersson M, Katila T, 2006: Motility and plasma membrane integrity of spermatozoa in fractionated stallion seminal plasma. Reprod. Domest. Anim. 41 33-38. II Kareskoski AM, Reilas T, Sankari S, Andersson M, Güvenc K, Katila T, 2010: Alkaline and acid phosphatase, -glucuronidase, and electrolyte levels in fractionated stallion ejaculates. Reprod. Domest. Anim. 45 e369-e374. III Kareskoski AM, Rivera del Alamo MM, Güvenc K, Reilas T, Calvete JJ, Rodriguez-Martinez H, Andersson M, Katila T, 2010: Protein composition of seminal plasma in fractionated stallion ejaculates. Reprod. Domest. Anim. 46 e79-e84. IV Kareskoski AM, Sankari S, Johannisson A, Kindahl H, Andersson M, Katila T: The association of the presence of seminal plasma and its components with stallion fertility and sperm longevity in fractionated ejaculates. Submitted for publication. 9 Abbreviations ACP acid phosphatase AP alkaline phosphatase AV artificial vagina AUC area under the curve BG -glucuronidase BSP bovine seminal protein CASA computer assisted sperm analysis CFDA carboxyfluorescein diacetate CRISP cysteine-rich seminal protein DFI DNA fragmentation index DIFF difference between two samples GPX glutathione peroxidase HIGH ejaculate fraction with the highest sperm concentration HSP horse seminal protein KE Kenney’s extender (skim milk extender) KMT KE supplemented with a modified Tyrode’s medium LIN linearity LOW ejaculate fraction with the lowest sperm concentration MMP matrix-metalloproteinase MW molecular weight NAG -N-acetylglucosaminidase PGE2 prostaglandin E2 PGF prostaglandin F PI propidium iodide PMI plasma membrane integrity PMN polymorphonuclear cell PMOT progressive sperm motility PRE pre-sperm fluid PSA prostate-specific antigen PSP porcine seminal protein RAP percentage of rapid sperm cells ROS reactive oxygen species RP-HPLC reverse-phase high performance liquid chromatography SCSA sperm chromatin structure assay SDS-PAGE sodiumduodecyl-sulphate polyacrylamide gel electrophoresis SOD superoxide dismutase SP seminal plasma SP1 sample stored with seminal plasma present SP0 sample stored without SP STR straightness 10 TI TMOT VCL VSL WE 2D-PAGE testicular index total sperm motility average sperm velocity straight-line sperm velocity whole ejaculate two-dimensional polyacrylamide gel electrophoresis 11 1. Introduction Seminal plasma (SP) is the fluid portion of semen, secreted by the epididymides and the accessory glands shortly before and during ejaculation. It is a fluid composed of several different types of compounds, such as proteins, ions, amino acids, monosaccharides, lipids, polyamines, prostaglandins and steroid hormones. The main function of seminal fluid is to act as a vehicle that flushes the urethra and simultaneously propels spermatozoa through the stallion’s genital tract during ejaculation. Further functions include the transport, protection and nutrition of spermatozoa in the female genital tract; seminal factors also regulate sperm capacitation, the acrosome reaction and gamete interaction (reviewed by Töpfer-Petersen et al. 1998). The research presented in this thesis provides in part a new look into an old area of investigations, as many of the studies on the SP of domestic animals date back to the 1960s and 1970s. Although SP has been the subject of research for decades, the available information still seems lacking, partly because of the immense species-specific variation (Aumüller et al. 1990, Massanyi et al. 2003) in reproductive physiology, making extrapolations between species a minefield. Recently, progress has been made especially in the fields of proteomics and genomics, but the physiological role of SP is still not fully understood. While artificial insemination using cooled transported semen has become an integral part of modern horse breeding, enabling international semen transport and a wider choice of stallions available for mare owners, an increased need for reliable and practical techniques for both breeding soundness evaluation and semen processing has emerged. As sperm longevity and stallion fertility vary markedly between individuals, it is of interest for the equine industry to investigate the factors producing these effects. Stallion SP influences the function and survival of ejaculated sperm during in vitro storage and in the female genital tract, and some of the stallion variation could perhaps be explained by inherent differences in the composition of SP. Knowledge of the composition of SP is needed in order to develop semen handling procedures and to perhaps even predict the storage tolerance of spermatozoa. The differences in the composition of SP between consecutive parts of the ejaculated fluid may also provide information on the sequence of fluid release from the accessory sex glands during ejaculation. The following literature review is partly based on previous reviews by Kareskoski and Katila (2008) and Katila and Kareskoski (2006), and presents research on selected aspects of stallion reproductive physiology, mainly on the sequence of ejaculation and on the composition of SP and the effects of SP on sperm longevity and pre-fertilization events. Compared to humans and other animal species, fairly limited data are available on stallion SP, and research on other mammalian species is therefore also discussed. 12 2. Review of the literature 2.1. Ejaculation of the stallion and characteristics of stallion semen A reproductively sound stallion expresses normal sexual behavior and produces an adequate number of progressively motile, morphologically normal spermatozoa, at least 1 x 109 spermatozoa at any time of the year (Kenney et al. 1983). A stallion with normal libido shows an immediate interest in the mare and mounts her within just a few minutes. The mean reaction time (the time from visual contact with the mare to mounting) has been reported to be 221 s, and the mean number of mounts was 1.8 mounts/ejaculate (Pickett et al. 1976). Excessive sexual preparation, teasing, or false mounts increase the amount of SP and the total volume of semen, without increasing the sperm output (Ionata et al. 1991). Emission of fluids from the cauda epididymis, ductus deferens and the accessory sex glands occurs before ejaculation, through smooth muscle contractions in these organs. The emission reflex is under -adrenergic control, involving sympathetic fibres from the lumbosacral segment of the spinal cord. Ejaculation is a series of 6-9 jets, produced by rhythmic contractions of the ischiocavernosus, bulbospongiosus, and urethralis muscles, and this process is controlled by the pudendal nerve and the sacral segment of the spinal cord (reviewed by Tibary 2007). The ejaculatory jets differ in sperm concentration, semen volume and biochemical composition. Before the actual ejaculation, a clear and watery pre-sperm fluid is secreted. As this often occurs before intromission, this pre-sperm fluid is probably not present in large amounts in collected semen or in the uterus after ejaculation. The first three jets, the sperm-rich fractions, contain ¾ of the total number of sperm, even though these fractions represent less than half of the total ejaculate volume. The volume of semen and the sperm concentration in each of the jets decrease towards the end of the ejaculation (Tischner et al. 1974, Kosiniak 1975, Varner et al. 1987). Horses are long-day seasonal breeders, and seasonal differences are seen in the amount and composition of SP. The seasonal effects on seminal plasma seem more pronounced than the effects on sperm characteristics (Gebauer et al. 1976, Abou-Ahmed et al. 1993). Seasonal variation has been detected in the semen volume, total sperm count, seminal pH, and in sexual behavior depicted by the reaction time and mounts per ejaculate (Pickett et al. 1976). The volume of semen, sperm count and sperm motility of fresh semen have been shown to be higher in summer than in winter (Janett et al. 2003). These effects could be associated with changes in sex steroid levels, which vary according to the season in both blood and SP (Braun et al. 1996, Hoffmann and Landeck 1999). However, the correlations between the chemical characteristics of SP and plasma concentrations of testosterone have been reported to be weak (Gebauer et al. 1976). 13 In a study on over 550 ejaculates, the mean volume of gel-free semen in stallion ejaculates was 58.4 ml, ranging from 44.8 ml in March, to 80.8 ml in July. The mean volume of gel was 7.1 ml for first ejaculates and 2.0 ml for second ejaculates collected one hour after the first ejaculation. First ejaculates contained 281 x 106 sperm/ml compared with 170 x 106 sperm/ml in second ejaculates. The total amount of spermatozoa was 14.7 x 109 in first ejaculates and 7.9 x 109 in second ejaculates (Pickett et al. 1976). The endocrinological profile of stallions is also affected by disease, hard physical exercise (Janett et al. 2005) and medications, such as dexamethasone, which increases the total protein and iron concentrations, and decreases the alkaline phosphatase (AP) levels of SP (Danek 2001). 2.2. Anatomy and function of the accessory glands The stallion has a full set of accessory glands (i.e. paired bulbourethral glands, ampullar glands, seminal vesicles and a prostate gland), as opposed to some of the other domestic animal species. All of the accessory glands are also present in the bull and in man, but there are marked differences between species in the amount of secretions produced by these glands, reflected in ejaculate volumes ranging from 2–8 ml in the bull to 50–150 ml in the stallion. Dogs do not have seminal vesicles or bulbourethral glands, and boars have prominent seminal vesicles and bulbourethral glands, but lack ampullae (Nickel et al. 1979). Because of these fundamental species differences, comparison between species is rarely fruitful because the seminal fluid varies in composition, as the gland contributions vary. Even if there are macroscopic similarities in some of the accessory glands between different species, the composition of the produced secretions is often not comparable (Beyler and Zaneveld 1982). The ampullae are enlargements of the distal part of the vas deferens, and they have been presumed to function as a storage site of spermatozoa, but also in the transport of spermatozoa through the urethra during ejaculation (Nickel et al. 1979). The ampullae are quite large in the stallion compared with other species, and they contain a variable amount of yellowish, creamy fluid. Ergothioneine in SP has been found to originate in the ampullae (Mann et al. 1956). Some absorption, such as transport of proteins through pinocytosis, can take place in the ampullae, but the secretory functions are better documented. The stallion’s ampullae secrete several different types of compounds, e.g. proteins, Na+ and K+ ions, water, ergothioneine, glycerylphosphorylcholine and monosaccharides, and a variety of glycosidases, such as -N-acetylglucosaminidase and mannosidase (Beyler and Zaneveld 1982). The seminal vesicles of the stallion are pear-shaped sacs with muscular walls lined with a thick mucous membrane. Each seminal vesicle is 10–15 cm long and has a diameter of 3–6 cm (Nickel et al. 1979). Seminal vesicle secretions are transparent or slightly opaque and viscous fluid (Mann et al. 1956). In many other species, the seminal vesicles secrete large amounts of fructose and ergothioneine, whereas citric acid and lactic acid are more 14 prominent in the stallion (Mann et al. 1956, Beyler and Zaneveld 1982). The prostate gland contains only a small amount of fluid (Mann et al. 1956). The prostate gland has two nodular lobes connected over the midline by an isthmus. The prostate and the seminal vesicles are united by connective tissue, and they are thus difficult to palpate transrectally (Nickel et al. 1979). The two bulbourethral glands are club-shaped and situated at the pelvic outlet, dorsolateral to the urethra (Nickel et al. 1979). The composition of SP in the consecutive ejaculate fractions varies, as the accessory glands release their contents in a specific order during the emission of fluid into the pelvic urethra and the subsequent ejaculation. This sequence has been studied by identifying certain markers for particular accessory gland secretions in different ejaculate fractions. An early study by Mann et al. (1956) showed that the first sperm-rich ejaculate jets contained mainly spermatozoa and ampullar secretions high in ergothioneine, while the latter spermpoor fractions contained vesicular secretions high in citric acid. Carnitine, glycerylphosphorylcholine and choline have later been used as markers for the epididymides, the N-acetyl function of glycoproteins and spermine for ampullae, acetic acid for the bulbourethral glands, citric acid for seminal vesicles, and lactic acid for prostate secretions (Magistrini et al. 1995). Magistrini et al. (2000) used an automated semen collection device (Lindeberg et al. 1999) to collect fractionated ejaculates and 1H magnetic resonance spectroscopy to quantify these markers of accessory gland secretions. Bulbourethral fluid was secreted first (pre-sperm fluid), and then epididymal and ampullar secretions formed the first sperm-rich fractions of the ejaculate, while seminal vesicle fluid appeared by the end of the ejaculation. The concentration of acetic acid did not differ between the ejaculate fractions, suggesting that it does not serve as a specific marker for the bulbourethral glands. Similar results on the sequence of ejaculation have been shown earlier using transrectal ultrasonography. Ampullar and prostatic activity began prior to the start of ejaculation, with fluid release from the prostate continuing throughout the first urethral contractions. The seminal vesicles did not release their contents until the end of prostatic secretion (Weber and Woods 1993). There is always a certain amount of overlap between the fractions, and the secretions from the different glands do not appear by themselves at any point during the ejaculation (Mann et al. 1956). 2.3. Semen collection and semen processing for storage Several different models of artificial vaginas (AVs) are available for semen collection in stallions. These AVs are either open-ended, with semen collection into a separate receptacle, or closed, with semen collection into a bottle attached to the AV (Love 1992). Fractionated ejaculates can be collected using an open-ended AV, although modifications of closed AVs such as the Missouri model can also facilitate the collection of ejaculatory fractions. Collection of only the sperm-rich fractions with an open-ended AV can be beneficial, especially in stallions producing semen with a low sperm concentration (Tischner et al. 1974), as it will render centrifugation unnecessary. Low sperm 15 concentrations are problematic when semen is prepared for cooled storage, because adequate dilution of semen ( 1:4 = semen:extender) recommended to achieve adequate sperm longevity (see section 2.5.) would result in too low sperm concentrations (Varner et al. 1987). A more elaborate form of fractioning is achieved using a computer-controlled phantom such as the Equidame® (Haico Oy, Loimaa, Finland), where the ejaculate is collected in several cups according to a pre-set target weight of each cup corresponding to a particular semen volume. The Equidame® phantom is equipped with a detachable open-ended AV, a metallic funnel, and a semen collection tray with 5 plastic cups. The computer-controlled tray moves horizontally as the cups reach their target weight during the ejaculation (Lindeberg et al. 1999). After the collection of semen, any gel is promptly removed, the semen volume is recorded and a sample is taken to determine the sperm concentration. For cooled storage, prewarmed extender (+37 ºC) is then added to lower the volume proportion of SP. The final volume is calculated based on the sperm concentration. To lower the volume proportion of SP in stored semen, more extender is added once the sperm concentration is known and the needed volume can be calculated. A total number of 500–1000 x 106 progressively motile spermatozoa, with a concentration of 25–100 x 106 spermatozoa/ml, is often considered an adequate insemination dose for semen transport that lasts less than 24 h (Katila 1997, Blanchard et al. 2003). The volume as such has no influence on maintenance of motility of fertility, but for practical reasons, such as the limited size of the commonly used Styrofoam transport boxes, the total volume of the insemination dose usually varies between 10–40 ml. The role of SP, extenders and sperm concentration during cooled storage (+5 °C) of semen is discussed in section 2.5. Because of the limited volume available in the plastic straws (0.25 to 2.5 ml) used for storing frozen semen, the superfluous and possibly harmful SP has to be removed before semen freezing in order to reduce the semen volume. To this end, semen is diluted with extender, centrifuged, and the supernatant is removed. Because semen is not washed by repeated centrifugations, a small amount of SP (< 5%) will be left, even though the semen has been centrifuged. The main proportion of SP, however, is removed before freezing stallion sperm in liquid nitrogen (Blanchard et al. 2003). 2.4. The composition of stallion seminal plasma 2.4.1. Enzymes Several enzymes have been identified in stallion accessory glands and in SP, and efforts have been made to study the correlations of enzyme activities with semen quality and stallion fertility. The origin of a few of the SP enzymes in the various reproductive organs 16 and accessory glands has been identified, but in many cases the physiological function of these compounds has remained elusive. Alkaline phosphatase (AP) is a widely distributed phosphomonoesterase (reviewed by Coleman 1992). There are high levels of AP activity in the SP of reproductively normal stallions, and spermatozoa are not a significant source of the enzyme. As AP activity is mainly derived from the testes and epididymides, measurement of AP can be used to differentiate ejaculatory failure and excurrent duct blockages from testicular azoospermia (Turner and McDonnell 2003). In dogs, AP activity is similarly found in the epididymides and the seminiferous tubules (Frenette et al. 1986, Kutzler et al. 2003), and can be used as a marker for ejaculation and tubular patency (Kutzler et al. 2003, Stornelli et al. 2003). Activity of AP has been found in seminal vesicle fluid in bulls and boars, and in prostatic secretions in many mammals (Beyler and Zaneveld 1982). High levels of acid phosphatase (ACP) are found in the prostatic fluid of man, monkeys and dogs, and lower levels are found in bull prostate fluid. The two phosphomonoesters, AP and ACP, differ in their pH optima and in substrate specificity, but both hydrolyze different types of phosphate monoesters (Beyler and Zaneveld 1982, Kramer and Hoffman 1997). The levels of acid phosphatase (ACP) activity reflect prostate function in dogs (James et al. 1979), and increased levels of ACP are associated with prostate cancer in humans (Mahan and Doctor 1979, Tanaka et al. 2004, Tappel 2005). In the stallion, studies on ACP have been limited to epididymal tissue (Lopez et al. 1989). The levels of both AP and ACP in SP are positively correlated with the sperm count and negatively with semen volume (Pesch et al. 2006). Both AP and ACP are under androgenic control (Beyler and Zaneveld 1982). In the boar, AP levels in seminal plasma vary according to the season (Kozdrowski and Dubiel 2004). Several different types of glycosidases have been identified in mammalian SP. Stallion seminal vesicle secretion contains -glucuronidase (BG), -glucosidase, -glucosidase, galactosidase, -galactosidase and -N-acetylglucosaminidase (NAG), and the ampullae are a significant source of NAG and -mannosidase (Beyler and Zaneveld 1982). In stallion SP and in the epididymides, -1,4-glucosidase activity has been detected, and the activity levels have been observed to increase from the proximal caput epididymis to the cauda epididymis. The acid form of the enzyme was predominant in the cauda epididymis, while the neutral form dominated in the caput epididymis and in SP (Dias et al. 2004). Stallion epididymal fluid also contains NAG (Fouchécourt et al. 2000). In humans, -galactosidase, BG and NAG bind to the surface of the sperm head in the epididymal duct and may be involved in modifying the sperm surface before fertilization (Sosa et al. 1991, Barbieri et al. 1994, Barbieri et al. 1996), with NAG also participating in the acrosome reaction (Brandelli et al. 1994). Together with other enzymes, acrosomal BG acts on glycosaminoglycans and participates in causing cumulus dispersion (Rethinaswamy et al. 1994). Despite their essential function in the fertilization process, the over-expression of certain glycosidases is associated with subfertility and oligo-astheno-teratozoospermia in men (Corrales et al. 2000, Corrales et al. 2002). In the bull, low sperm concentrations and morphologically abnormal sperm are seen in conjunction with decreased levels of 17 NAG, -fucosidase, Perttula 1986). -galactosidase, BG and -mannosidase (Jauhiainen and Vanha- Lipocalin-type prostaglandin D2 synthase and the angiotensin-I-converting enzyme have been shown to be strongly correlated with stallion fertility, whereas the total protein concentration in SP was not (Barrier-Battut et al. 2005). Lipocalin-type prostaglandin D2 synthase activity has been found in stallion epididymal fluid (Fouchécourt et al. 2000). Lipase activity has been demonstrated in stallion seminal plasma, and it is negatively correlated with sperm motility (Carver and Ball 2002). Other enzymes demonstrated in equine seminal plasma include three carbonic anhydrase isoenzymes detected in the seminal vesicles, prostate and bulbourethral glands. These enzymes appear to regulate the bicarbonate concentration and pH of seminal plasma (Asari et al. 1996). Angiotensinconverting enzyme has been found in the plasma membranes of ejaculated and epididymal spermatozoa and in postpubertal testes. The influence of angiotensin II on sperm motility has also been evaluated and it affected some motility parameters, such as the average path velocity (VAP) and curvilinear velocity (VCL), but not total sperm motility (TMOT) (Ball et al. 2003). Pesch (2005) measured the activities of lactate dehydrogenase, aspartate aminotransferase, glutamate dehydrogenase, gamma-glutamyltransferase and creatine kinase in stallion SP. Of the mentioned enzymes, only lactate dehydrogenase was correlated with motility, the sperm count and volume. Platelet-activating factor (PAF) acetylhydrolase activity has also been detected in the semen of several mammalian species, including the stallion. Sperm-derived PAF stimulates sperm motility and is involved in capacitation (Hough and Parks 1994). In humans, the levels of matrix metalloproteinases (MMPs) are correlated with semen quality characteristics such as sperm concentration, morphology and motility (Baumgart et al. 2002, Buchman-Shaked et al. 2002). The two gelatinases, MMP-2 and MMP-9, and their pro-forms are present in human seminal plasma (Shimokawa et al. 2002). Proteinases and proteinase inhibitors have been characterized in different regions of the epididymis in rams, boars and stallions, and one of the main metalloproteinases from the caput epididymis of the ram and stallion was a pro-form of MMP-2. In all three species, antibodies against MMP-2, MMP-3, and MMP-9 and proteinase inhibitors were detected in epididymal fluids (Métayer et al. 2002). 2.4.2. Other protein compounds In addition to enzymes, the SP of most domestic animal species contains protein compounds similar to those present in blood plasma, such as prealbumin, albumin, -, and -globulins, transferrin and immunoglobulins (Polakoski and Kopta 1982). The suggested functions of SP proteins include their involvement in several essential steps preceding fertilization, such as regulating capacitation, establishment of the oviductal sperm reservoir, modulation of the uterine immune response, and sperm transport in the 18 female genital tract, and also in gamete interaction and fusion (reviewed by TöpferPetersen et al. 2005). Calvete et al. (1994) described eight types of proteins among stallion SP proteins, and these were named and numbered horse seminal protein (HSP)-1 to HSP-8 according to the order of appearance of peaks on the reverse-phase liquid chromatography charts. Most of the proteins in SP were of low molecular weight (14-30 kDa) and formed multi-protein aggregates. All of the 8 protein groups, except HSP-4, were found to attach to the sperm surface. The two major proteins, HSP-1 and HSP-2, accounted for 70-80% of the total protein. They were shown to be heparin-binding proteins, and hypothesized to modulate capacitation. In fractionated ejaculates, the amount of total protein is lowest in pre-sperm fluid, and highest in the sperm-rich fractions (Koskinen et al. 2002). Structurally, the main proteins in stallion SP belong to three groups: proteins carrying fibronectin type II (Fn-2) modules, cysteine-rich secretory proteins (CRISPs), and spermadhesins. The most abundant SP proteins in many species, including the stallion, are short Fn-2 type proteins, such as HSP-1 and HSP-2 (also called SP-1 and SP-2). These proteins are similar to the major bovine heparin-binding proteins (BSP) associated with capacitation (reviewed by Töpfer-Petersen et al. 2005). At the time of ejaculation, the Fn-2 type proteins typically bind to sperm membrane phospholipids with a phosphorylcholine head group, preferentially phosphatidylcholine or sphingomyelin, causing changes in the membrane structure (Greube et al. 2004, Ekhlasi-Hundrieser et al. 2005). The ampullae are the major source of HSP-1 and HSP-2, while a larger Fn-2 type protein (EQ-12) is mainly secreted in the epididymis (Saalmann et al. 2001), but expression of Fn-2 type proteins occurs along the entire genital tract of stallions (Ekhlasi-Hundrieser et al. 2005). A member of the CRISP family in stallion SP is HSP-3 (equine CRISP-3), primarily produced in the ampullar glands and in the seminal vesicles (Magdaleno et al. 1997, Schambony et al. 1998). An association of polymorphism of the CRISP3 gene with stallion fertility has been suggested (Hamann et al. 2007). Recent findings suggest that CRISP-3 is the protein responsible for the selective protection of live spermatozoa from polymorphonuclear (PMN) cell binding and allows the sperm to survive and travel to the oviduct, despite an ongoing breeding-induced endometritis (Troedsson et al. 2010). In stallions, the protein profiles of whole ejaculates have been characterized in a few studies. In sodium dodecyl-sulphate polyacrylamide gel electrophoresis (SDS-PAGE), Frazer and Bucci (1996) found 14 protein bands and described a protein profile typical for the stallion. Seven of these bands were present in all of the samples, but the relative amounts of each protein varied between individual stallions. The findings of a twodimensional (2D)-PAGE study by Brandon et al. (1999) were similar, with 14 protein groups common in all stallions. Four of these proteins (named SP-1, SP-2, SP-3 and SP-4) were correlated to the breeding scores of the stallions. The breeding score was calculated by dividing the number of conceptions per cycle by the number of breedings for each stallion for four successive breeding seasons. Based on molecular mass, it was 19 hypothesized that SP-3 and SP-4 are similar to the two major proteins found in the 2DPAGE study by Frazer and Bucci (1996) and to HSP-1 and HSP-2 identified by Calvete et al. (1994). Three proteins (SP-2, SP-3 and SP-4) were found in higher concentrations in stallions with low fertility scores, while SP-1 was positively correlated with fertility and was suggested to be homologous to a bovine fertility-associated protein described by Killian et al. (1993). Proteins associated with high fertility in bovine SP are osteopontin, derived from the ampulla and the seminal vesicle (Cancel et al. 1997), and prostaglandin D2 synthase originating in the testis and epididymis (Gerena et al. 1998). Protein profiles in 2D-PAGE were compared between SP from stallions exhibiting high and low fertility by Jobim et al. (2005). One protein band (protein number 19) was detectable only in ejaculates from stallions in the high fertility group, whereas the amount of another protein (number 17) was higher in ejaculates of stallions with low fertility. The authors suggested that protein spot 17 could correspond to CRISP-3. Of the other stallion SP proteins described by Calvete et al. (1994), HSP-4 was presumed to be related to a calcitonin gene-like product. Calcitonin levels are correlated with sperm motility in the semen of infertile men (Mungan et al. 2001). In the horse, HSP-7 has been identified as the only member of the spermadhesin family, and like its porcine homologue AWN-1, it shows zona-pellucida-binding activity (Reinert et al. 1996). Hoshiba and Sinowatz (1998) detected synthesis of the AWN protein homologue in the testes, the ductus epididymis and the seminal vesicles of stallions. In the boar, the non-heparinbinding spermadhesins PSP-I/PSP-II are of special interest because of their effects on sperm function. These spermadhesins have a dose- and time-dependent beneficial effect on the viability of highly extended boar spermatozoa, whereas the heparin-binding proteins in porcine SP decrease sperm viability at high extension ratios (Centurión et al. 2003). The supplementation of PSP-I/PSP-II in the freezing extender does not affect post-thaw sperm survival (Cremades et al. 2004), and it has a deleterious effect on the ability of boar spermatozoa to penetrate oocytes in vitro (Caballero et al. 2004). In the study by Calvete et al. (1994), HSP-6 and HSP-8 appeared to be different isoforms of a kallikrein-like protein homologous with human prostate-specific antigen. 2.4.3. Prostaglandins Early studies have shown that prostaglandin E 1 and E2 (PGE2) are present in human SP, and in larger quantities than other prostaglandins (Jonsson et al. 1975). Claus et al. (1992) verified the presence of prostaglandin F (PGF ) in stallion semen. No differences in the levels of the immunosuppressive PGE2 were detected between groups of fertile and infertile men in a study by Camejo (2003), but an earlier paper reported lower PGE2 levels in oligoterato-asthenozoospermic patients with genital infections compared with fertile controls (Huleihel et al. 1999). The transport of spermatozoa may be influenced by PGE2, as it controls oviductal smooth muscle function (Weber et al. 1991), but it is not known whether SP-derived PGE 2 could 20 reach the uterotubal junction at levels high enough to have any effect on oviductal muscle. The infusion of PGE2 into the proximal end of the uterine horn prior to insemination improved the pregnancy rate in mares bred by a stallion with good quality semen, but did not affect pregnancy rates in mares bred by a stallion with poor quality semen (Woods et al. 1999). Other effects of PGE2 include the modulation of the immune response in the female genital tract, and it may also influence sperm function more directly. Both SP and PGE2 stimulate the release of the proinflammatory neutrophil chemotactic factor interleukin-8 (IL-8) and the anti-inflammatory cytokine interleukin-10 (IL-10) from peripheral blood and monocytes, suggesting both anti- and proinflammatory effects of SP (Denison et al. 1999). In rams, PGE2 levels are inversely related to the capacity of sperm to undergo the acrosome reaction (Archbald et al. 1990). Porcine SP also contains components that regulate the synthesis of prostaglandins in the oviduct (Kaczmarek et al. 2010). 2.4.4. Electrolytes and trace elements Calcium is one of the most extensively studied components of mammalian semen, as it is involved in many physiological events surrounding sperm function and fertilization. Extracellular Ca2+ regulates sperm capacitation through decreasing the availability of intracellular ATP (Baker et al. 2004). Hyperactivation of sperm (Marquez and Suarez 2004) and induction of the acrosome reaction are also mediated, at least in part, by Ca2+. Abnormal levels of ionized Ca2+ can induce a premature acrosome exocytosis and lead to a decrease in fertility (Yanagimachi and Usui 1974). Spontaneous acrosome exocytoses are also associated with increased concentrations of ionized Ca2+ in stallion SP (Pesch 2005). As stated earlier, notable species differences exist in the composition of SP. About 60-75% of the total amount of Ca is ionized (as Ca2+) in stallion SP (Pesch et al. 2006), whereas in human semen, only 2-4% is in the ionized form (reviewed by Owen and Katz 2005). These marked species differences make the extrapolation of results from other species of questionable use, but some research on human reproduction will be presented here, since knowledge of the composition of SP, and the consequences of this variation, in domestic animal species is incomplete. It has been shown in humans that post-thaw motility values are lower with increased levels of Ca2+ (Meseguer et al. 2004), but the levels of total Ca in SP did not vary between fertile and subfertile men in a study by Wong et al. (2001). In stallions, Barrier-Battut et al. (2002) reported individual variation in the concentrations of Ca, Mg and Cu, but not of Zn, in sperm-rich fractions, but these differences did not explain the variation in post-thaw motility parameters. In bulls, post-thaw motility values increased when MgCl2 was added to the freezing extender (Lapointe et al. 1996). In ram semen, Ca and Mg concentrations were higher in sperm than in SP, and both components were positively correlated with the sperm concentration (Abdel-Rahman et al. 2000), as opposed to the negative correlation that is seen in stallions. In the stallion, the concentrations of total Ca and ionized Ca2+ were positively correlated with ejaculatory volume, indicating 21 the accessory sex glands as the main contributors to Ca in SP (Pesch et al. 2006). In humans, the levels of Ca and Mg have also been suggested to reflect the secretory function of the accessory glands, because the concentrations of these components were lower in men with accessory gland infections (Adamopoulos and Deliyiannis 1983, Edorh et al. 2003). The addition of K to semen extenders seems to improve motility of stallion and human sperm, but the effect depends on the proportion of SP retained in the sample during storage (Padilla and Foote 1991, Karow et al. 1992). The ionic composition and osmolarity of SP are not correlated (Rossato et al. 2002). In sheep, increasing potassium levels are negatively correlated with progressive motility, while the reverse is true for sodium and chloride (Abdel-Rahman et al. 2000). Significant stallion variation has been reported in Na, K, Ca and Cl levels (Amann et al. 1987). There is some evidence that the concentrations of Cu and Fe in stallion SP vary between normal samples and samples with decreased semen quality as assessed with routine semen evaluation methods (Pesch 2005). The concentrations of Fe, Zn, and Cu are negatively correlated with semen volume, with Fe and Zn concentrations also correlating with the sperm concentration (Pesch et al., 2006). Massanyi et al. (2003) assessed the concentrations of various trace elements in the SP of several animal species. Zinc and Fe levels were positively correlated in stallion and bull semen, and boar semen contained more Zn than semen from stallions or bulls. In addition, Cu and Fe concentrations were higher in ram semen compared with stallions and boars. Sperm-chromatin stability in human ejaculates is Zn-dependent, and Zn levels can also affect pregnancy rates (as reviewed by Björndahl and Kvist 2003). In human semen samples, high Zn concentrations were associated with a decrease in progressive motility, but Zn concentrations did not affect the time to pregnancy (Sorensen et al. 1999). 2.4.5. Other components studied in stallion seminal plasma The secretions of the accessory glands contain many low molecular weight organic constituents such as citric acid, glycerylphosphorylcholine, glucose, glycerol, glycerylphosphorylinositol, inositol, lactic acid, sorbitol and small amounts of fructose (Polakoski and Kopta 1982). The carbohydrate content in stallion SP differs from that of bulls, with a considerably lower amount of free carbohydrates in stallions (Gebauer et al. 1976). Some inositol and glucose are found in stallion SP, but only negligible amounts of fructose and no galactose. Galactose is the main type of bound carbohydrate in stallion seminal plasma, but glucose, mannose and fucose have also been detected (Baronos et al. 1971). Stallion SP also contains hormones, ergothioneine, small amounts of ammonia and urea, free amino acids, and several different forms of lipids (Polakoski and Kopta 1982). The hormonal content of SP is beyond the scope of this text. Variation between species in 22 sperm membrane composition may be related to the cold shock sensitivity of sperm. Cholesterol is the main sterol in the membranes of boar, bull, stallion, and rooster spermatozoa, and choline and ethanolamine phosphoglycerides and sphingomyelin are the major types of phospholipids (Parks and Lynch 1992). Stallion semen also contains prostasome-like particles with a characteristic lipid profile and high amounts of cholesterol and sphingomyelin (Arienti et al. 1998). These membrane vesicles are mainly found in the sperm-rich part of the ejaculate, but their origin and function are not well known (El-Hajj Ghaoui et al. 2004), although effects on sperm motility, the acrosome reaction and the immune system have been proposed (reviewed by Sostaric et al. 2008). Oxidative damage to sperm is of significance for sperm survival during storage. Spermatozoa are protected against oxidative damage caused by reactive oxygen species (ROS), such as hydrogen peroxide, mainly by catalase, glutathione peroxidase (GPX) and superoxide dismutase (SOD). Cryodamaged or morphologically abnormal sperm, and also neutrophils, are significant sources of ROS (Ball et al. 2001, Baumber et al. 2002). Limited GPX- and SOD-activity exists in sperm, and thus the main protective activity is due to the protective enzymes in SP (Baumber and Ball 2005). The prostate gland is likely to be the main source of catalase in the reproductive tract of the stallion (Ball et al. 2000). In fractionated stallion ejaculates, catalase is found in high amounts in all fractions of the ejaculate, except for in the pre-sperm fluid (Koskinen et al. 2002), which could indicate that the prostate contributes to several ejaculatory fractions or that catalase also originates in other glands. The testes and epididymides are the main sources of GPX (Fouchécourt et al. 2000). Sperm motility is reduced by ROS, (Baumber et al. 2000), and they also cause DNA fragmentation and membrane damage in spermatozoa (Baumber et al. 2003) due to lipid peroxidation (Ball et al. 2000). It is likely that increased ROS production is responsible for these effects, rather than a decrease in the amount or function of the ROS protective enzyme systems (Zini et al. 1993). Despite an apparent physiological function of these enzymes, adding antioxidant to the freezing extender does not seem to improve sperm survival during storage (Baumber et al. 2005). The production of ROS may have a physiological role in promoting capacitation and tyrosine phosphorylation (Baumber et al. 2003), involved in the signalling pathway controlling capacitation and the acrosome reaction (Leyton et al. 1992). In a review by Griveau and Le Lannou (1997), it was concluded that small amounts of ROS are needed for normal sperm function, but excessive ROS production is highly toxic to sperm. 2.5. The effects of seminal plasma on sperm during storage Reducing the amount of SP in cooled semen has been considered beneficial for sperm survival during storage, and this can be accomplished through centrifugation and 23 subsequent removal of the supernatant, extension of semen, or through collecting only the sperm-rich fraction of the ejaculate. Several studies have shown that when the volume proportion of SP is lowered to less than 5 or 10%, sperm motility is better maintained compared to samples containing a higher proportion of SP (10–30%), during both cooled storage and freezing and thawing (Jasko et al. 1991, Braun et al. 1994, Todd et al. 2001, Alghamdi et al. 2002). A high SP content (40–50%) in cooled semen during storage for 24–48 h was shown to be detrimental to sperm motility (Jasko et al. 1991), whereas a proportion of 20% SP did not have any negative effects on semen quality during storage for 24 to 48 h (Jasko et al. 1992). Lower SP proportions (1.25%, 2.5% and <10%) seem to maintain sperm motility even better (Pruitt et al. 1993, Todd et al. 2001). The removal of SP before semen storage also had beneficial effects on DNA integrity compared to samples containing 20% SP during cooled storage in a study by Love et al. (2005). The removal of SP has also been shown to increase the resistance to hypo-osmotic shock, and to reduce the responsiveness to induced acrosome exocytosis (Barrier-Battut et al. 2010). Short term (15 min) exposure of sperm to SP did not reduce post-thaw semen quality, but longer periods of exposure (2, 4 or 6 hours) of sperm to 5% or 20% SP in the freezing extender had negative effects on sperm (Moore et al. 2005). The proportion of SP included in insemination doses did not affect pregnancy rates when semen was stored at +5 °C for less than 12 h (Katila et al. 2010a). In a recent study, the sperm concentration did not affect sperm survival when the effect of the proportion of SP was eliminated. Sperm were stored at +5 °C for 24 h at sperm concentrations of 25 or 250 x 106 sperm/ml and a SP content of 20%, and no differences between the samples were detected in total sperm motility, progressive sperm motility, membrane integrity or DFI (Voge et al. 2010). The choice of extender affects the results of storage trials, depending on whether the samples contain SP or not. There is some evidence that the commonly used skim milk extender (Kenney et al. 1975) may not be the best alternative for cooled storage of semen after complete SP removal. Padilla and Foote (1991) compared sperm motility values of centrifuged and non-centrifuged semen samples stored 4 ºC for 24–72 h in either skim milk extender (KE) containing glucose, non-fat dry skim milk, Na penicillin, streptomycin and water, or KE supplemented with a modified high-potassium Tyrode’s medium (KMT). This extender contained 65% KE extender and 35% modified Tyrode’s medium, which consisted of NaCl, KCl, NaHCO3, NaH2PO4, Na lactate syrup, CaCl2. MgCl2, HEPES, Na pyruvate, bovine serum albumin and deionized water. The osmotic pressure and pH for KE and KMT were 330 and 315 mOsm/kg and 6.8 and 7.2, respectively. The Na+ and K+ content in SP (120 and 26 mM) was higher than in KMT (43 and 10 mM) and KE (1 and 1 mM). When SP was removed after centrifugation, the motility values were higher in the samples that were stored in KMT compared with the samples in KE, but when SP was present (in samples extended to a sperm concentration of 50 x 106), KE seemed superior to KMT. In another study, KMT gave higher motility values after storage than KE, irrespective of the presence of SP (Rigby et al. 2001). The KMT extender together with removal of SP also improved the longevity of sperm motility compared with KE in a more recent study (Akcay et al. 2006). 24 The effect of SP on sperm longevity in the different ejaculate fractions has been examined in a few studies. Sperm-rich fractions extended to a sperm concentration of 25 x 106 sperm/ml showed superior motility values compared with whole ejaculates and lower extension rates (100 x 106 and 50 x 106; Varner et al. 1987). Sieme et al. (2004) found that centrifuged sperm-rich fractions had higher post-thaw motility and viability compared with both non-centrifuged sperm-rich fractions (i.e. samples containing SP) and centrifuged whole ejaculates. Thus, it seems that the fluid in the last ejaculate fractions, mainly produced by the seminal vesicles, has a more pronounced negative effect on sperm survival. Vesiculectomy has been shown to improve sperm motility and viability values after 4 h of cooled storage compared to semen collected before surgery from the intact stallions (Webb et al. 1990). On the other hand, Akcay et al. (2006) reported that SP from the sperm-rich fraction decreased progressive sperm motility and plasma membrane integrity (PMI) compared with SP from the sperm-poor fractions. Variation between stallions in the effects of SP on spermatozoa is evident. It has been reported that spermatozoa from individual stallions react differently to the presence or removal of SP, and to the choice of extender (Padilla and Foote 1991, Jasko et al. 1992, Akcay et al. 2006). Exchange studies, where spermatozoa from one stallion were stored in another stallion’s SP, have also been carried out; the effects of SP varied between stallions, and SP from some of the stallions seemed to have less negative effects on sperm survival during storage (Aurich et al. 1996). On the other hand, adding autologous or heterologous SP to cooled semen did not affect sperm motility at 24 or 48 h in a study by Todd et al. (2001). It has been recommended that stallions producing semen with poor tolerance to cooling and storage should be evaluated for the effects of their SP and different extenders on semen quality, thus enabling alterations of semen processing techniques for individual stallions (Brinsko et al. 2000, Love et al. 2005). Brinsko et al. (2000) studied the effect of SP on sperm motility during cooled storage in stallions considered “good coolers” and “bad coolers”, grouping the stallions according to the reduction in progressive sperm motility after 24 h of cooled storage. Centrifugation and partial removal of SP (90%) increased the percentage of progressively motile spermatozoa of “bad coolers” after 48 h of cooling. At 24 h the effect was not as obvious. The authors concluded that components of SP adversely affect sperm motion characteristics after cooling and storage. These effects are independent of their concentration, and some stallions will benefit from centrifugation and SP removal, especially if the semen is stored > 24 h. 2.6. The effects of seminal plasma in the genital tract of the mare A transient inflammatory reaction, caused mainly by the presence of sperm, occurs after natural breeding and artificial insemination (Kotilainen et al. 1994), and SP contains factors that modulate this process. In in vitro experiments, SP has been shown to suppress chemotaxis of (PMNs), opsonization and phagocytosis of spermatozoa and complement 25 activity (Troedsson et al. 1999, Troedsson et al. 2000, Troedsson et al. 2005). The suppression of PMN chemotaxis was caused to a large degree by a factor with a molecular weight in the range of 50–100 kDa, but other SP components may also affect PMN chemotaxis (Troedsson et al. 1999). The presence of SP in insemination doses seems to protect spermatozoa within the uterus, perhaps through a reduction in the binding of spermatozoa to PMNs (Troedsson et al. 2002). A protein fraction <35 kDa has been shown to suppress PMN binding to both viable and dead sperm, and a greater molecular weight fraction promotes binding between PMN and dead sperm (Troedsson et al. 2005). Subsequently, the SP protein CRISP-3 has been shown to be responsible for the protection of live spermatozoa from PMN binding (Troedsson et al. 2010). In contrast, in vivo experiments have shown a stimulatory effect of SP on the inflammatory response and an increase in the number of PMNs in the uterus after insemination compared with extender or saline (Kotilainen et al. 1994, Parlevliet et al. 1997). Pregnancy rates have, however, been shown to be equal in normal mares inseminated with or without SP (Portus et al. 2005). Effects of SP on sperm transport have been described in swine. The number of spermatozoa found in the oviducts 4 h after insemination was higher when the spermatozoa had been thawed in SP instead of a buffer (Einarsson and Viring 1973). The presence of SP also affects the blood flow to the uterus and oviducts in mares (Bollwein et al. 2001). The functions of SP in the sperm-uterus interaction and fertilization do not directly correspond to fertility rates. When mares were inseminated with semen stored without SP or with semen containing 20% SP, there were no differences between the pregnancy rates of the two mare groups (Rigby et al. 2001). 2.7. Research objectives Based on earlier research on the effects of SP on spermatozoa during storage, it was hypothesized that the removal of SP would clearly improve sperm longevity. As reviewed here, research on the effects of SP on sperm survival during storage has mainly been carried out on whole ejaculates, or the sperm-rich fractions have been compared to whole ejaculates. The studies presented in this thesis compared the effects of the presence of SP from different ejaculate fractions on sperm survival during storage. The goal was to improve semen handling procedures in order to achieve better sperm longevity and fertility. The information on the composition of SP has also been incomplete, and further studies were needed to examine the differences in composition between different SP fractions, and how the differences in the composition of SP between stallions are linked to sperm survival during storage. 26 3. Aims The studies forming this thesis focused on the composition of fractionated stallion SP and on the effects of ejaculatory fractions on sperm survival during storage in an attempt to examine the physiology of stallion SP and how it affects sperm longevity and fertility. The ultimate goal was to provide a sound basis for improved methods of storing stallion semen. The specific aims of these studies were to: 1. determine which SP fraction could best maintain sperm survival during storage (I, IV); 2. study whether the removal of most of the SP before storage would improve sperm survival during storage (I, II); 3. analyze and compare the levels of selected enzymes, electrolytes and proteins in different SP fractions (II, III, IV); 4. assess stallion variability in the effects of SP and in the composition of SP (I, II, III); and 5. examine the association between the components of SP and semen quality, sperm longevity and fertility (IV). 27 4. Material and methods Presented here is an overview of the material and methods used in each study, with an overview of the experiments summarized in Table 1. More detailed descriptions are available in the original publications (I–IV). Five experiments (Experiments A–E) were conducted to collect data for the four original publications, each including one, two or three trials that were parts of different experiments (A-E). In Experiment A, the purpose was to collect semen and SP samples to evaluate the effects of the presence of SP and different ejaculate fractions on sperm survival during cooled storage. In Experiment B, the effects of SP and the different ejaculate fractions on sperm survival were studied after freezing and thawing. Experiment C was designed to compare the effects of SP from two fertile and two subfertile stallions on sperm survival during cooled storage in different ejaculate fractions. Seminal plasma was also exchanged between stallions. In Experiment D, SP samples were collected for the determination of the levels of selected biochemical components in the different fractions. Similar SP samples were also collected in Experiments A, C, and E. In Experiment E, samples were collected for analysis of the association between components of SP and semen quality, sperm longevity, and fertility. 28 29 II: Trial 2 C (Feb 2003) III: Trial 2 II: Trial 3 E IV (Aug 2006) D (Feb 2005) 1, 5, 6 I: Trial 2 B (Oct 2001) III: Trial 1 1, 5, 6, 7 A I: Trial 1 (Aug 2001) II: Trial 1 43 stallions Sperm motility and DFI after cooled storage in semen fractions stored and with or without SP; Na, Cl, K, Mg, Ca, Pi, AP, ACP, PGE2 in SP fractions SDS-PAGE of SP fractions Katila et al. 2001, Koskinen et al. 2002 Published abstracts 1-4 fractions/ejaculate: HIGH: fraction with highest sperm concentration; LOW: fraction with lowest sperm concentration Kareskoski et al. 2008a, Kareskoski et al. 2008b 1-4 fractions/ejaculate: Kareskoski et al. HIGH: fraction with highest sperm 2009 concentration; LOW: fraction with lowest sperm concentration; WE: whole ejaculate 1-4 fractions/ejaculate: Kareskoski et al. PRE: pre-sperm fluid; 2005b HIGH: fraction with highest sperm concentration; LOW: fraction with lowest sperm concentration; WE: whole ejaculate Kareskoski et al. 2005a, Katila et al. 2006 1-3 fractions/ejaculate: Katila et al. 2002 HIGH: fraction with highest sperm Katila et al. 2003 concentration; LOW: fraction with lowest sperm concentration Cups 1 and 2: sperm-rich fractions; Cups 3 and 4: sperm-poor fractions; WE: whole ejaculate Cup 1: pre-sperm fluid; Cups 2 and 3: sperm-rich fractions; Cups 4 and 5: sperm-poor fractions; WE: whole ejaculate Fractioning Proteins (RP-HPLC, mass spectrometry) in SP 1-3 fractions/ejaculate: fractions HIGH-1 and HIGH-2: sperm-rich fractions; LOW: sperm-poor fractions; WE: whole ejaculate Na, Cl, K, Mg, Ca, Pi, AP, ACP, BG in SP fractions Sperm motility and PMI after freezing and thawing in semen fractions stored with or without SP Sperm motility and PMI after cooled storage in semen fractions stored with or without SP; Na, Cl, K, Mg, Ca, Pi, AP, ACP, BG in SP fractions Analyses 1, 2, 4, 5, 8, Na, Cl, K, Mg, Ca, Pi, AP, ACP, BG in SP 9, 10 fractions 1, 2, 3, 4 Stallion identity Experiment Original (time) article Table 1. Overview of experiments, material, methods and published abstracts. 4.1. Animals The stallions in Experiments A-D were housed at the Equine Research Station at MTT Agrifood Research Finland in Ypäjä. The stallions in Experiment E were privately owned breeding stallions, and semen was collected at the stud farm where the stallion was placed for the breeding season. 4.1.1. Experiment A (I: Trial 1, II: Trial 1) and Experiment B (I: Trial 2) Four reproductively normal stallions were used for semen collection. Three of the stallions (Finnhorses, Stallions 1 and 5, aged 14 and 17 years, and one Standardbred, Stallion 7, aged 15 years) had previously been used for breeding, while the third Finnhorse stallion (Stallion 6, aged 7 years) had no breeding history. After one week of daily semen collections, the total sperm counts on the first day of Experiment A were 5.8 x 109 spermatozoa (Stallion 1), 10.0 x 109 spermatozoa (Stallion 5), 7.4 x 109 spermatozoa (Stallion 6), and 5.2 x 109 spermatozoa (Stallion 7). 4.1.2. Experiment C (II: Trial 2, III: Trial 1) Semen was collected from two stallions that were considered to be reproductively normal and had good breeding records, with seasonal pregnancy rates of 80% and 82% in the previous season (Stallion 1: Finnhorse aged 16 years; Stallion 3: Standardbred aged 12 years), and two stallions producing semen with a low sperm concentration and low progressive motility and considered to be subfertile (Stallion 2: Finnhorse aged 19 years; Stallion 4: Finnhorse aged 24 years). The book of stallions 2 and 4 had to be limited because of subfertility: Stallion 2 and Stallion 4 served only three and eight mares in 2002, and thus, fertility data are not meaningful. Stallion 2 was the son of Stallion 4. A breeding soundness examination was performed on all of the stallions before collecting semen samples for analyses. The testicular index (TI, Kenney et al. 1983) was calculated based on measurements of the testes, and the sperm motility of an extended fresh semen sample was assessed subjectively using a phase contrast microscope (Olympus BH2, Olympus Optical Co., Hamburg, Germany). At the time of the examinations, Stallion 1 had a TI of 5.9 and progressive sperm motility of 50%, and the corresponding figures for Stallion 3 were 4.9 and 60%. Stallion 2 had a TI of 5.7 and motility of 20%, and Stallion 4 correspondingly 1.9 and 30%. After one week of daily semen collections, the total daily sperm counts were 5.5 x 109 spermatozoa (Stallion 1), 2.0 x 109 spermatozoa (Stallion 2), 4.5 x 109 spermatozoa (Stallion 3), and 1.3 x 109 spermatozoa (Stallion 4). 30 4.1.3. Experiment D (II: Trial 3, III: Trial 2) Semen samples were collected from seven Finnhorse stallions (ages 3–26) varying in breeding history. 4.1.4. Experiment E (IV) Semen was collected from 43 stallions (ages 4–23 years) at nine stud farms in Finland. A total of 30 stud farms, housing at least one stallion that had served over 20 mares in the previous season, were invited to offer their residing stallions to participate, and all of the available stallions were included in the study. All of the samples were collected during the breeding season in 2006. Twenty stallions were Standardbred trotters (ages 4–23 years), 17 were Finnhorses (ages 8–23 years), four were warmblood riding horses (ages 9–19 years) and two were ponies (ages 8 and 9 years). The breeding history and current use of the stallions was variable, with the stallions serving 2 to 150 (median 14) mares in 2006. 4.2. Semen collection The semen collections in Experiments A, B, C and D (I–III) took place at the equine research station in Ypäjä (MTT Agrifood Research Finland), using a computer-controlled fractioning phantom (Equidame®, Haico Oy, Loimaa, Finland) that collects portions of the ejaculate separately into five cups (Fig. 1). In this collection device, the fractioning is based on a pre-set target weight of each cup, and the settings can be adjusted individually to optimize the separation of fractions. The stallion ejaculates into the metallic funnel that is in front of the AV inside the phantom (Fig. 2). The plastic cups are attached to a moving tray and are located below the metallic funnel, so that semen fractions can be collected into the plastic cups that move horizontally below the funnel (Lindeberg et al. 1999). Before the actual experiments, the stallions were collected daily to empty the extra-gonadal sperm reserves. Fig. 1. A fractionated ejaculate collected with the Equidame®phantom. Pre-sperm fluid is seen in the first cup on the right, followed by sperm-rich and sperm-poor fractions. The last small cup and the large cup contained mostly gel in this ejaculate. 31 Fig. 2. The Equidame® phantom consists of the following parts: a detachable open-ended artificial vagina (1A) and a rubber ring (1B) inside the vagina, a detachable metallic funnel (2), 5 detachable metallic holders (3A) and 5 plastic cups (3B), small styrofoam shields (3C) that envelope the plastic cups to isolate semen, and a computer control panel for adjusting the settings for semen fractioning (Lindeberg et al. 1999). 4.2.1. Experiment A (I: Trial, II: Trial 1) Each stallion was collected twice a week for two weeks in two trials. In Trial 1, pre-sperm fluid without any spermatozoa was collected in cup 1, the sperm-rich fractions into cups 2 and 3, and the last fractions with a low sperm concentration into the last two cups (combined in cup 4). Small amounts of pre-sperm fluid may also have been included in cup 2, which contained the first jets of the ejaculate. 4.2.2. Experiment B (I: Trial 2) Cup 1 contained sperm-rich fractions, and the following cups (2–5 cups per ejaculate) contained fractions with successively decreasing sperm concentration. Pre-sperm fluid was not collected separately in this trial. 4.2.3. Experiment C (II: Trial 2, III: Trial 1) Semen was collected twice a week for two weeks. The fractioning phantom collected the ejaculates in 4–5 cups (Table 2). 32 Table 2. Semen volume (ml; mean, min-max) and sperm concentration (106 spermatozoa/ml; mean, min-max) in fractionated ejaculates collected in 3 cups (Experiment C). Volume Stallion Cup 1 Sperm concentration Cup 2 Cup 3 Volume Sperm concentration Volume Sperm concentration 1 15.5 194.5 13.8 238.8 7.8 15.3 2 (5-24) 9.5 (5-16) (160-253) 90.8 (54-154) (9-21) 15.5 (10-25) (135-340) 154.3 (103-213) (4-12) 11.0 (7-16) (11-20) 44.8 (4-95) 3 9.0 (8-10) 294.0 (273-315) 9.5 (8-11) 147.0 (158-441) 4.7 (4-5) 234.3 (86-273) 4 10.8 (9-14) 13.3 (5-26) 34.3 (20-41) 24.3 (8-45) 16.3 (10-21) 6.5 (2-13) In Trial 2 (II), the cups with the highest (HIGH) and lowest (LOW) sperm concentration were selected from each ejaculate for the SP analyses. In Trial 1 (III), cup 1 contained presperm fluid and the first sperm-rich jets (portion 1 of the ejaculate, HIGH-1), while Cup 2 contained the rest of the sperm-rich part of the ejaculate (portion 2 of the ejaculate, HIGH2). The following cups (3–5) were fractions with a low sperm concentration, and they were combined to form portion 3 of the collected ejaculate (LOW). The HIGH-fractions always preceded the LOW fractions by definition. 33 4.2.4. Experiment D (II: Trial 3, III: Trial 2) Semen was collected twice a week for three weeks. The fractioning phantom collected the ejaculates in 4–5 cups, as in Experiment C (Table 3). The cups with the highest (HIGH) and lowest (LOW) sperm concentration were selected from each ejaculate for the SP analyses, and pre-sperm fluid (PRE) samples were collected separately. Table 3. Semen volume (ml; mean, min-max) and sperm concentration (106 spermatozoa/ml; mean, min-max) in all fractionated ejaculates collected in Experiment D, and the total sperm count (109 spermatozoa) for each stallion after one week of daily ejaculations. Stallion Total sperm count Fraction Sperm concentration Volume 1 8.3 HIGH LOW 317.7 (294-380) 110.5 (12-223) 14 (8-20) 7.3 (5-10) 2 6.2 PRE HIGH LOW 142.5 (108-172) 48.7 (17-115) 11.5 (5-20) 19.7 (8-26) 12.0 (5-27) 4 0.8 PRE HIGH LOW 13.0 (8-20) 2.8 (2-5) 12.2 (8-20) 25.8 (23-28) 18.3 (6-34) 5 8.0 PRE HIGH LOW 319.0 (281-387) 174.7 (76-279) 2.5 (2-3) 8.6 (6-13) 5.3 (4-6) 8 8.5 PRE HIGH LOW 238.2 (175-323) 67.8 (15-159) 7.0 (4-14) 17.3 (10-28) 9.8 (2-15) 9 7.4 PRE HIGH LOW 98.0 (12-194) 359.0 (212-422) 9 8.7 (2-18) 13.7 (10-25) 10 2.4 HIGH LOW 204.5 (177-232) 171.0 (153-189) 8.5 (7-10) 6.5 (4-9) PRE: pre-sperm fluid; HIGH: fraction with the highest sperm concentration; LOW: fraction with the lowest sperm concentration. 4.2.5. Experiment E (IV) One ejaculate was collected from each stallion. Ejaculatory jets were collected as 1–4 fractions depending on individual differences in the ejaculates. The ejaculates were collected using either an open-ended artificial vagina (AV), a modified closed AV or the Equidame® phantom, depending on stallion preference and stud farm. The Equidame® 34 phantom was available only at one stud farm (6 stallions). The open-ended and closed AVs were from variable manufacturers, because the AV available at the stud farm was preferably used. The modified closed AV (8 stallions) was a Missouri type AV with an ultrasound probe cover (Kruuse A/S, Langeskov, Denmark) attached at the end of the AV instead of the semen collection bottle, and the same probe covers were also attached to the funnel used in semen collection with the open-ended AV. Using this technique, the ejaculate fractions could be separated with hemostatic forceps or by hand, as the fractions could be seen entering the probe cover (Fig. 3). The sperm concentrations (measured with a Bürker counting chamber) in the HIGH and LOW fractions collected with the different methods in Experiment E (IV) are shown in Table 4. Fig. 3. The fractioning method used in Experiment E (IV). The ejaculate fractions were separated with forceps or by hand as the fractions could be seen entering the probe cover. Table 4. The sperm concentrations (106 spermatozoa/ml; median, min – max) in the HIGH and LOW fractions collected with an open-ended AV (29 stallions), a Missouri-type closed AV (8 stallions) and the Equidame®-phantom (6 stallions). Semen collection method Open-ended AV Closed AV Equidame®-phantom All methods Fraction: HIGH 300.5 (24-933) 259.0 (30-429) 288.0 (38-463) 300.0 (24-983) 35 Fraction: LOW 54.0 (6-522) 85.0 (21-280) 12.0 (7-88) 54.0 (6-522) As a description of the collected ejaculates, the initial sperm motility and sperm morphology is shown in Table 5. The methods of motility and morphology assessment are described in section 4.4. Table 5. Sperm morphology (%; median, min-max) and sperm motility (%; median, min-max) before storage in Experiment E. Sperm morphology Normal sperm Abnormal heads Abnormal acrosomes Tailless heads Proximal droplets Distal droplets Abnormal midpieces Bent tails Coiled tails Median Min-max 52.5 5.0 4.0 2.5 5.5 12.5 2.0 3.0 3.0 2.5-82.0 0.5-41.5 0.0-11.0 0.0-83.0 1.0-24.5 0.0-42.0 0.0-27.5 0.5-10.0 0.0-43.5 80 50 5-90 0-80 Sperm motility Total motility Progressive motility 4.3. Semen handling and preparation of samples After collection, a percentage (5–10% depending on the study) of the volume of each cup was combined to form a sample representing the whole ejaculate (WE) (I, II, III). 4.3.1. Experiment A (I: Trial 1, II: Trial 1) Trial 1 in original article I compared the effects of different SP fractions on the maintenance of sperm motility and membrane integrity during cooled storage. The semen was extended in a ratio of 1:1 with skim milk extender (Kenney et al. 1975). Half of each sample was centrifuged (500 g, 15 min) and the supernatant removed. Extender was added to the centrifuged samples to a sperm concentration of 50 x 106 sperm/ml after the removal of supernatant. The other half of the sample was not centrifuged, but immediately diluted to a final sperm concentration of 25–50 x 106 sperm/ml, with a semen to extender ratio of 1:1–3. All of the samples were stored for 24 h and transported to the laboratory in 1.5-ml vials in an Equitainer transport container (Hamilton-Thorne Research, South Hamilton, MA, USA), which maintains a temperature of 5–10 ºC for 48 h (Katila et al. 1997). After storage, the samples were incubated for 5 min at 37 ºC prior to the sperm motility and PMI analyses. 36 To separate SP, a part of all samples were centrifuged at 3000 g for 20 min, and filtered through a 0.45 µm filter (Minisart, Sartorius, Göttingen, Germany). The filtered SP was frozen in 1-ml aliquots at -75 ºC. 4.3.2. Experiment B (I: Trial 2) Skim milk extender (Kenney et al. 1975) was added to all cups at a ratio of 1:1, and the samples were placed in a refrigerator at 5 ºC for 2 h to increase the effects of SP. After centrifugation at 500 g for 15 min, the supernatant was removed and skim milk extender with or without SP was added. Egg yolk and glycerol (2% and 2.5% of the final volume, respectively) were also added. The contents of each cup were extended further to a final concentration of 200 x 106 sperm/ml by adding either more of the mentioned freezing extender, or freezing extender and a part of the previousl y removed supernatant (50% SP and 50% extender). The samples were frozen in 0.5-ml straws, at a rate of -50 ºC/min from +13 ºC to -140 ºC using a programmable freezer (Planer Kryo 10 Series II, Sunbury of Thames, UK) and plunged into liquid nitrogen. The straws were thawed for 30 s in a 37 ºC water bath, and the samples were then diluted by adding skim milk extender at a ratio of 1:1, and the incubation at 37 ºC was continued for an additional 15 min. 4.3.3. Experiment C (II: Trial 2, III: Trial 1) After semen collection, HIGH-2 was divided into two parts. Half of the semen in HIGH-2 was extended 1:1 using skim milk extender (Kenney et al. 1975) and centrifuged at 500 g for 15 min. The supernatant was removed and the sperm pellet was re-suspended in a small volume of skim milk extender. The sperm concentration was then determined using a Bürker counting chamber, and the suspension was extended with skim milk extender to a final concentration of 75 x 106 spermatozoa/ml. Semen was subsequently mixed in a ratio of two parts extended semen to one part SP (2:1, v/v). The final sperm concentration was 40–50 x 106 spermatozoa/ml. A part of each sample was also suspended in extender and SP from another stallion, as SP was exchanged between Stallions 1 and 2, and Stallions 3 and 4. The samples were packed in an Equitainer (Hamilton Research, Inc., South Hamilton, MA, USA) in 1.5-ml vials and stored for 24 h while shipped to the laboratory. The fractions HIGH-1, LOW, WE, and the remaining half of HIGH-2 were processed further to be used as SP. The SP samples were centrifuged at 3000 g for 20 min, and filtered through a 0.45 µm filter (Minisart, Sartorius, Göttingen, Germany). The filtered SP was frozen in 1-ml aliquots at -75 ºC. 4.3.4. Experiment D (II: Trial 3, III: Trial 2) All of the samples were centrifuged at 3000 g for 20 min, and filtered through a 0.45 µm filter (Minisart, Sartorius, Göttingen, Germany). The filtered SP was frozen in 1-ml aliquots at -75 ºC. 37 4.3.5. Experiment E (IV) After semen collection, the gel was removed and the volume of each fraction was measured. The sperm concentration in each fraction was determined using a Bürker counting chamber. One drop of semen was placed on two glass slides and smears for morphological evaluation were prepared and air-dried. For the sperm chromatin structure assay (SCSA) to be performed at a later date, a sample of 2–10 x 106 spermatozoa from each fraction of raw semen was pipetted into cryovials and TNE-buffer (9.48 g Tris-HCl, 52.6 NaCl, 2.23 g disodium-EDTA, aqua ster. ad 600 ml, pH 7.4) was added (ad 1.5 ml in each vial). The SCSA samples were placed in liquid nitrogen vapor (3 cm above the liquid surface) for 10 min, and then plunged into liquid nitrogen. Each fraction was divided into two parts: one half was centrifuged for preparation of SP (SP samples), and the other half was processed for cooled storage (semen samples). The semen samples were extended in a semen to extender ratio of 1:1 using skim milk extender, and total and progressive sperm motility were assessed subjectively using phase contrast microscopy. Each fraction of the semen samples was divided into two centrifuge tubes, and centrifuged at 500 g for 10 min. After removal of the major part (about 5% was left) of the supernatant (i.e. extender and SP), one part of each fraction was extended to a concentration of 50 x 106 sperm/ml using only skim milk extender (SP0), and the other part was extended to the same sperm concentration using a combination of supernatant and skim milk extender at a SP to extender ratio of 1:2 (SP1). The semen samples were stored in 1-ml vials at 5 °C for 24 h. After 24-h cooled storage, 5 x 106 spermatozoa from each semen sample were pipetted into cryovials for SCSA analyses, TNE buffer was added (ad 1.5 ml in each vial), and the samples were placed in liquid nitrogen vapor (3 cm above the liquid surface) for 10 min and plunged into liquid nitrogen. The SP samples in Experiment E were centrifuged at 4000 g (15 min), and the supernatant was filtered using 0.45 µm filters (Millex-HV, Millipore, Billerica, MA, USA). Protease inhibitor (Trasylol 10000 Kallikrein Inactivator Units (KIU)/ml, Bayer Schering Pharma, Berlin, Germany) was added to each sample (500 KIU/ml of SP) to reduce the proteolytic activity on enzymes and other proteins. The SP samples were stored frozen in 1-ml aliquots at -75 °C until analyzed. 4.4. Sperm quality assessment Sperm motility, plasma membrane integrity and morphology were examined in the laboratory of the Department of Production Animal Medicine at the Faculty of Veterinary Medicine (University of Helsinki). 4.4.1 Sperm motility (I, III, IV) The samples containing spermatozoa were warmed in a water bath (37 ºC, 5 min) after storage for 24 h. In Experiment A, the sperm motility parameters total motility (TMOT), 38 progressive motility (PMOT), average path velocity (VAP) and the percentage of rapid cells (RAP) were determined with a Hamilton-Thorne Motility Analyzer (HTM-S, version 7.2., Hamilton-Thorne Inc, MA, USA). In Experiments C and E, the motility parameters TMOT, PMOT, VAP, curvilinear velocity (VCL), straightness (STR) and linearity (LIN) were evaluated using the SpermVision CASA system (SpermVision Minitüb, Tiefenbach bei Landshut, Germany). 4.4.2. Plasma membrane integrity (PMI) (I) The membrane integrity of the stored spermatozoa was assessed with the method described by Harrison and Vickers (1990), using staining with carboxyfluorescein diacetate and propidium iodide (CFDA/PI) (Molecular Probes Inc., OR, USA) and counting 200 cells in fluorescence microscopy. 4.4.3. Sperm morphology staining and evaluation (IV) The morphology smears were fixed and stained using the Giemsa method according to Watson (1975), and 200 sperm were assessed in each sample. The morphological characteristics were classified in the following way: morphologically normal spermatozoa, abnormal heads, abnormal acrosomes, abnormal midpieces, tailless heads, proximal cytoplasmic droplets, distal cytoplasmic droplets, bent tails and coiled tails. 4.4.4. Sperm chromatin structure assay (IV) Sperm chromatin stability of sperm from both the raw and stored semen samples was measured as the susceptibility of sperm DNA to denaturation with the sperm chromatin structure assay (SCSA) at the Department of Anatomy, Physiology and Biochemistry, Swedish University of Agricultural Sciences (SLU, Uppsala, Sweden) as described by Evenson et al. (1999). Acridine orange was added to each sample after exposure to an acid detergent solution that induces partial DNA denaturation in situ, and flow cytometry was then used to measure either red-colored (abnormal spermatozoa, denatured DNA) or greencolored (normal sperm) fluorescence. The sperm DNA fragmentation index (DFI), formerly referred to as Cells Outside the Main Population (COMP t), was assessed by calculating the amount of red fluorescence divided by the total (red plus green) fluorescence, indicating the amount of denatured sperm DNA in relation to the total DNA in each sperm cell. 4.5. Determination of enzyme and electrolyte levels in seminal plasma (II, IV) The enzyme and electrolyte levels were measured at the Central Laboratory of the Faculty of Veterinary Medicine at the University of Helsinki (Clinicum, Viikki). Levels of AP activity were measured according to the recommendations of the Scandinavian Society for 39 Clinical Chemistry and Clinical Physiology (SCE 1974), and total ACP using the method described by Hillman (1971). The enzyme activities were measured using the reagents and adaptations for serum applied by the manufacturer of the automatic chemistry analyser (KONE Pro Selective Chemistry Analyser, Thermo Fisher Scientific, Vantaa, Finland). Analyses of BG were performed on microtitration trays. Activity levels of BG were analyzed by the method described by Linko-Löppönen and Mäkinen (1985) using 4methyl-umbelliferyl- -glucuronide (Sigma M-9130, Sigma Chemical Co., St Louis, MO, USA) as substrate and measuring the resulting fluorescence (Fluoroskan Ascent, Labsystems, Helsinki, Finland). Colorimetric methods were used for the determination of Ca (Bauer 1981), Mg (Gindler and Heth 1971) and Pi (Daly and Ertigshausen 1972) and concentrations of Na+, K+ and Clwere measured directly by ion selective electrodes. The analyses were performed on the same automatic chemistry analyzer that was used for the enzyme analyses. The concentration of prostaglandin E2 (PGE2) was determined with a commercial enzyme immunoassay kit (Correlate-EIA, Assay Designs Inc., Ann Arbour, MI, USA) according to the manufacturer’s specifications. The PGE2 determinations were performed by Prof. Hans Kindahl at SLU in Sweden. 4.6. Protein analyses of seminal plasma (III) In Experiment C (III: Trial 1), peptides were isolated as described by Calvete et al. (1994) by reverse-phase high-performance liquid chromatography (RP-HPLC) on an RP-100 Lichrospher (Merck, Germany) C18 column (25 x 4 mm, 5- m particle size) eluted at 1 ml/min with a gradient of 0.1% trifluoroacetic acid in water and acetonitrile. N-terminal amino acid sequence analyses were performed using an Applied Biosystems 472 automated protein sequencer (Applied Biosystems, Langen, Germany) to identify HSP-1, HSP-2, and HSP-4 in relation to the RP-HPLC curves. The area-under-the-curve (AUC) on the chromatograms was used for comparing the relative amounts of proteins within each fraction. Samples of SP were also analyzed using matrix-assisted laser desorption ionization – time of flight (MALDI-TOF) mass spectrometry. The RP-HPLC and mass spectrometry were performed at the Institute of Biomedicine (CSIC) in Valencia, Spain. In Experiment D (III: Trial 2), the SP samples were analyzed by SDS_PAGE electrophoresis in 12% polyacrylamide gels. The molecular weight (MW) was estimated using a protein standard (Precision Plus Protein All Blue Standard 161-0373, Bio-Rad Laboratories, Hercules, CA, USA). Densitometry gave a quantitative estimate of each band (AlphaImager HP, Alpha Innotech, San Leandro, CA, USA). The SDS-PAGE was done at the Universidad Autonoma de Barcelona, Spain. 40 4.7. Fertility data (IV) Fertility data were provided by the stud farms and the central Finnish trotting and breeding association (Suomen Hippos ry). Pregnancy rates were calculated from all reported matings in the year 2006 for the stallions included in the study. All mares that foaled or aborted were considered pregnant. First cycle pregnancy rates were the main fertility parameter used for statistical comparisons, and were calculated separately for mares inseminated with fresh semen or transported semen using data obtained from the stud farms and Suomen Hippos ry. Inseminations using frozen semen were excluded from the study. 4.8. Statistical analyses 4.8.1. Experiments A-D (I, II, III) Repeated measures analysis of variance was used to analyze the differences between SP fractions and the whole ejaculate in sperm motility parameters, membrane integrity and sperm concentration, and the effects of centrifugation and SP removal on motility and membrane integrity in publication I. Nonparametric tests were used to compare the means in publications II and III. The Friedman test, followed by the Wilcoxon signed-rank sum test was used to analyze the differences between SP fractions in the levels of the measured components, and the Kruskal-Wallis test for several independent samples was used to evaluate the differences between stallions in each semen fraction. The Mann-Whitney U-test was used in Trial 2 of publication II to compare the means of HIGH to LOW ratios of the measured SP components of the two stallion pairs (fertile vs. subfertile). Spearman’s rank correlation coefficient was used to evaluate the relationships between the measured components of SP and sperm concentration in Trial 2 of publication II, and the association between sperm concentration, total motility (TMOT), progressive motility (PMOT), and average path velocity (VAP) to the AUC of the peaks containing HSP-1, HSP-2, and HSP-4 in Trial 1 of publication III. Spearman’s rank correlation coefficient was also used in Trial 2 of publication III to study the correlation between sperm concentration and relative band density percentages. 41 4.8.2. Experiment E (IV) In Experiment E (IV), semen fractions with the highest (HIGH) and lowest (LOW) sperm concentration within each ejaculate were included in the statistical analyses. The differences between fractions in the levels of ACP, Na, K, and Cl, and in the mean difference (DIFF) in sperm motility parameters between SP1 and SP0 samples were evaluated using a two-sample t-test. The t-test was also used to compare mean values in sperm motility and DFI between fractions within the storage groups (SP1 and SP0), and between the storage groups within fractions. Because of the skewed distribution of the levels of PGE2, Ca, Pi and AP, and the mean DIFF in DFI, the nonparametric MannWhitney U-test was used for fraction comparisons. The sperm longevity parameters included in the correlation analyses were the DIFF in motility between SP1 and SP0 samples, and the DIFF in DFI between samples before and after storage (separately for SP0 and SP1 samples). The semen quality parameters were sperm concentration, total number of sperm, total and progressive motility before storage, and the number of morphologically normal sperm. The first cycle pregnancy rate depicted fertility. The correlations of sperm longevity, semen quality, and fertility parameters with the levels of the measured SP components were evaluated using the Pearson correlation coefficient for normally distributed parameters (all sperm motility parameters, the DIFF in motility between SP1 and SP0 samples in all fractions, the DIFF in DFI before and after storage with or without SP in WE, total number of sperm and number of morphologically normal sperm in all fractions, ACP in HIGH, Ca in WE, Cl, K, Mg, Na and first cycle pregnancy rate in all fractions, and AP in SR) and Spearman’s rank correlation coefficient for the other parameters. The dependent variables (i.e. the sperm longevity, semen quality, and fertility parameters) that correlated significantly with a minimum of two SP components were included in a stepwise linear regression analysis for the assessment of the association of the levels of these SP components (explaining variables) with each dependent variable. Significance was set at p < 0.05 in all studies. The statistical analyses in Studies I, II, and III were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). In Experiment E (IV), SAS software (SAS Institute Inc., Cary, NC, USA) was used. 42 5. Results 5.1. Effects of seminal plasma fractions and seminal plasma removal on sperm survival during storage (I, IV) 5.1.1. Effects of seminal plasma removal on sperm longevity (I, IV) In Experiment A (I), centrifugation and the subsequent removal of SP decreased all sperm motility parameters measured after 24 h of storage in all SP fractions, except for TMOT and PMOT in cup 4, containing the last fractions with a low sperm concentration, compared to samples containing SP. Sperm membrane integrity was not affected by centrifugation and SP removal. In Experiment E (IV), VAP, VCL, and VSL were significantly higher in SP1 than in SP0 in all fractions (Fig. 3). The DFI was higher in SP1 than in SP0 in HIGH and LOW, but not in WE. The DFI was higher in SP1 compared with samples taken before storage in all fractions, and the DFI was higher in SP0 compared with the samples taken before storage in LOW, but no differences were detected in HIGH or WE (Fig. 4). 43 SP1 200 SP0 Sperm motility * 150 * HIGH 100 * * * * 50 0 TMOT PMOT VAP VCL VSL SP1 Sperm motility 200 * 150 * 100 SP0 * LOW * * * 50 0 TMOT PMOT VAP VCL SP1 200 * Sperm motility VSL * 150 SP0 WE 100 * * * * 50 0 TMOT PMOT VAP VCL VSL Fig. 3. Mean (± SE) values of total motility (TMOT, %), progressive motility (PMOT, %), average path velocity (VAP, µm/s), curvilinear velocity (VCL, µm/s), and straight-line velocity (VSL, µm/s) in two cold-storage groups (with seminal plasma, SP1, and without seminal plasma, SP0) and in ejaculate fractions with high (HIGH) and low (LOW) sperm concentration, and in whole ejaculates (WE) in Experiment E (IV). *Asterisks indicate significant differences between SP1 and SP0 groups. 44 b 35 b c* 30 DFI 25 a a* b Before storage a a 20 SP1 after storage 15 10 SP0 after storage 5 0 HIGH LOW WE Fig. 4. Mean (± SE) values of the DNA fragmentation index (DFI) in ejaculate fractions with high (HIGH) and low (LOW) sperm concentration, and in whole ejaculates (WE), in samples taken before storage, and in two cold-storage groups (with seminal plasma, SP1, and without seminal plasma, SP0) in Experiment E (IV). abc Different letters indicate significant difference between storage groups (before storage, SP1 and SP0) within an ejaculate fraction. *Asterisks indicate significant differences between fractions within a storage group (SP1 and SP0). In HIGH, the DIFF between SP1 and SP0 (DIFF = SP1 – SP0) in sperm motility was positive (SP1 > SP0) in the following proportion of stallions (stallions with SP1 > SP0 / all stallions): TMOT 53%, PMOT 88%, VAP 65%, VCL 65%, STR 40% and LIN 53%. In LOW, the corresponding figures were: TMOT 57%, PMOT 46%, VAP 83%, VCL 86%, STR 26%, LIN 31% (Fig. 5). The DIFF between DFI after storage and before storage (DIFF = after storage – before storage) was positive (after storage > before storage) in 87% of stallions in HIGH, and in 91% of stallion in LOW (Fig. 6). The mean DIFF between SP1 and SP0 (DIFF = SP1 – SP0) samples in TMOT, PMOT, VAP, VCL, VSL and DFI was positive in both HIGH and LOW fractions. There was a positive mean DIFF in DFI between samples taken after storage and before storage (DIFF = after storage – before storage), in both SP1 and SP0 groups. A negative mean DIFF between SP1 and SP0 in STR and LIN was found in both HIGH and LOW fractions. In Experiment B (I), samples containing SP showed significantly higher post-thaw TMOT, PMOT and RAP in cup 1 (sperm concentration 234 ± 142 x 106) and cup 2 (sperm concentration 170 ± 96 x 106), and in WE, compared with samples stored in extender without SP. The presence of SP did not significantly affect VAP or membrane integrity. 45 In summary, the removal of SP decreased motility and decreased the amount of DNA damage during cooled storage. 5.1.2. Differences between seminal plasma fractions in the effects on sperm survival during storage (I, IV) In Experiment A (I), the differences between ejaculate fractions in the measured sperm motility parameters after cooled storage were non-significant. In Experiment E (IV), there were no significant differences between HIGH and LOW in any of the measured sperm motility parameters within the two storage groups (Fig. 3). In samples taken before storage, and in SP1, the DFI did not differ between HIGH and LOW. In SP0, the DFI was higher in LOW than in HIGH (Fig. 4). There were no significant differences between the two fractions in the mean DIFF of any of the motility parameters. The DIFF in DFI in the SP0 samples was lower in HIGH than in LOW, whereas no differences between fractions could be seen in the SP1 samples. After freezing and thawing in Experiment B (I), the mean values of TMOT, PMOT and RAP were significantly higher in cup 1 (the sperm-rich fractions) compared with WE. The differences between fractions in VAP and membrane integrity were non-significant. In summary, the least amount of DNA damage was found in the sperm-rich fraction stored without SP. Sperm motility after cooled storage did not differ between fractions. 5.1.3. Differences between stallions in sperm survival during storage (I) The differences between individual stallions were significant in several parameters in Experiments A and B (all motility values in Experiment A, and TMOT, PMOT, RAP and membrane integrity in Experiment B). The DIFF in total and progressive motility, and in straightness and linearity, between samples stored with or without SP (DIFF = SP1 – SP0) for each individual stallion in Experiment E (IV) is illustrated in Fig. 5 and 6, respectively. The DIFF in DFI between samples stored with or without SP (DIFF = SP1 – SP0) for each stallion in shown in Fig. 7. 46 30 Difference in motility (%) 25 HIGH 20 15 10 5 0 -5 1 2 3 4 5 6 7 8 9 10111213141516171819202122232425262728293031323334353637383940 -10 -15 Stallion -20 -25 Difference in total motility Difference in motility (%) 30 Difference in progressive motility LOW 20 10 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 -10 Stallion -20 -30 Difference in total motility Difference in progressive motility Fig. 5. The difference in total and progressive motility between samples stored with or without seminal plasma for each individual stallion in sperm-rich (HIGH) and sperm-poor (LOW) semen fractions in Experiment E (IV). 47 Difference in velocity (µm/s) 80 HIGH 60 40 20 0 1 2 3 4 5 6 7 8 9 10111213141516171819202122232425262728293031323334353637383940 -20 -40 Stallion Difference in average path velocity Difference in velocity (µm/s) 120 100 Difference in curvilinear velocity LOW 80 60 40 20 0 -20 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 -40 -60 -80 Stallion Difference in average path velocity Difference in curvilinear velocity Fig. 6. The difference in average path velocity and curvilinear velocity between samples stored with or without seminal plasma for each individual stallion in sperm-rich (HIGH) and spermpoor (LOW) semen fractions in Experiment E (IV). 48 HIGH Difference in DFI (%) 40 30 20 10 0 -10 -20 1 2 3 4 5 6 7 8 9 101112131415161718192021222324252627282930313233343536373839 Stallion Difference in DFI LOW Difference in DFI (%) 40 30 20 10 0 -10 -20 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 Stallion Difference in DFI Fig. 7. The difference in the DNA fragmentation index (DFI) between samples stored with or without seminal plasma for each individual stallion in sperm-rich (HIGH) and sperm-poor (LOW) semen fractions in Experiment E (IV). 5.1.4. The effects of seminal plasma exchange between stallions on sperm motility (III) In Experiment C, significant differences were seen between stallions in TMOT and PMOT after storage in the stallion’s own SP in HIGH-2. The SP of Stallion 3 seemed to reduce both the TMOT and PMOT of spermatozoa from Stallion 4, with a decrease of 14–28%, depending on the ejaculate fraction, compared with the samples stored in the stallion’s own SP. These differences were not tested statistically, because of the low number of samples (three or four ejaculates per stallion). 49 5.2. Levels of alkaline phosphatase, acid phosphatase, -glucuronidase and electrolytes in seminal plasma fractions (II, IV) 5.2.1. Differences between seminal plasma fractions (II, IV) The levels of AP and ACP were higher in HIGH than in LOW in all experiments. In Experiment D (II), the lowest levels of AP and ACP were found in PRE. The levels of BG were higher in HIGH than in LOW. The concentrations of Ca and Mg were higher in LOW than in HIGH, with the lowest concentrations found in PRE. The concentration of Pi was highest in HIGH and lowest in PRE in Experiment D (II), but in Experiment E (IV), the difference between fractions was non-significant. The K concentration did not differ between fractions in Experiment D (II), whereas in Experiment E (IV), the concentration of K was higher in LOW than in HIGH. The highest concentration of Na and Cl was found in PRE. There were no significant differences between HIGH, LOW, and WE in the concentration of Na and Cl in Experiment D (II), but in Experiment E (IV), the concentration of Na was higher in HIGH compared with LOW. The levels of PGE2 did not differ between fractions. The levels of the studied components in Experiments D and E are presented in Table 6. 50 Table 6. The levels (mean ± SE) of biochemical components of stallion seminal plasma in pre-sperm fluid (PRE), ejaculate fractions with the highest (HIGH) and lowest (LOW) sperm concentrations, and in whole ejaculates (WE). The studied components were prostaglandin E2 (PGE2), alkaline phosphatase (AP), acid phosphatase (ACP), Ca, Cl, K, Mg, Na and inorganic phosphate (Pi). N = number of samples. Experiment D (II) 7 stallions AP ACP Ca Cl K Mg Na Pi PRE HIGH LOW WE N = 16 N = 36 N = 33 N = 36 487 ± 114a 1.0 ± 0.2a 0.13 ± 0.01a 129.2 ± 0.6a 17.8 ± 0.6a 0.46 ± 0.03a 140.1 ± 0.6a 0.12 ± 0.01a 40374 ± 2906b 14.1 ± 1.4b 0.98 ± 0.07b 116.2 ± 2.3b 19.4 ± 0.6b 1.78 ± 0.17b 123.1 ± 2.8b 0.69 ± 0.04b 13738 ± 2501c 7.5 ± 1.1c 2.24 ± 0.26c 119.0 ± 1.3b 20.2 ± 0.8b 3.95 ± 0.55c 121.7 ± 2.2b 0.44 ± 0.04c 30396 ± 2719d 11.0 ± 1.1d 1.30 ± 0.10d 117.5 ± 1.3b 19.5 ± 0.6b 2.12 ± 0.11d 123.4 ± 1.9b PRE PGE2 AP ACP Ca Cl K Mg Na Pi Experiment E (IV) 43 stallions HIGH LOW N = 33 N = 32 303.6 ± 118.7 95052 ± 8331a 22.2 ± 1.8a 1.23 ± 0.16a 111.4 ± 1.3 19.5 ± 0.7a 1.83 ± 0.16a 125.0 ± 1.6a 0.59 ± 0.02 272.2 ± 98.1 30819 ± 5268b 11.1 ± 1.3b 3.90 ± 0.45b 114.7 ± 1.4 24.3 ± 1.6b 5.57 ± 0.67b 118.9 ± 2.6b 0.54 ± 0.04 0.60 ± 0.04d WE N = 29 383.3 ± 134.2 57743 ± 6868c 13.6 ± 1.8b 2.24 ± 0.19c 111.5 ± 4.1 20.0 ± 1.2a 3.10 ± 0.34c 121.6 ± 4.6ab 0.58 ± 0.03 Different superscripts within a row indicate a significant difference (p < 0.05). 5.2.2. Differences between stallions (II) The HIGH:LOW ratios of Pi and Cl varied significantly between the fertile and subfertile stallion pairs. In the HIGH fraction, there were significant differences between the two stallion pairs in the levels of ACP, BG, Pi and Cl, and in the sperm concentration. The stallion pair differences were non-significant in the LOW fraction in Experiment C. The levels of AP, ACP, BG, Na and K differed significantly between stallions in HIGH, LOW and WE, but not in PRE. The stallion differences in Ca, Pi and Cl concentrations 51 were significant in HIGH and WE, but not in PRE or LOW. The concentration of Mg varied between stallions only in HIGH in Experiment D. 5.2.3. Correlations of the levels of enzymes and electrolytes in seminal plasma with semen quality, sperm longevity, and fertility (II, IV) In Experiments C and D (II), the correlations between the measured components of SP and the sperm concentration in WE were significant. The significant correlations of the levels of SP components with semen quality parameters and sperm longevity detected in Experiment E (IV) are listed in Table 7. None of the measured SP components were correlated to the first cycle pregnancy rate in Experiment E. 52 Table 7. The association between levels of SP components and the listed variables (Experiment E (IV). Only variables with a significant correlations are listed in sperm-rich ejaculate fractions (HIGH), spermpoor fractions (LOW) and whole ejaculates (WE), and the correlation coefficient are shown for each significantly correlated variable. Non-significant correlations are not shown. The analyzed SP components were: alkaline phosphatase (AP), acid phosphatase (ACP), prostaglandin E2 (PGE2), inorganic phosphate (Pi), Ca, Cl, Mg and Na. The measured variables were: the mean difference (DIFF) in sperm motility in samples stored with SP (SP1) and without SP (SP0), the DIFF in the DNA fragmentation index (DFI) before and after storage in SP0 and SP1 samples, sperm concentration, the total number of sperm, total and progressive motility before storage, the number of morphologically normal sperm, and the first cycle pregnancy rate. *Marked variables were significantly associated with the dependent variable in the stepwise linear regression analysis. HIGH ( ) DIFF in motility Total motility Other variables WE ( ) PGE2 (-0.46) PGE2 (-0.41) PGE2 (-0.59) PGE2 (-0.54) - K (-0.51)* ACP (-0.45) ACP (-0.37) ACP (0.53) ACP (0.45) ACP (0.56) - PGE2 (-0.42) Mg (-0.46) Ca (-0.41) - - Ca (-0.49) - Sperm concentration AP (0.81)* ACP (0.67) Cl (-0.62) K (0.36) Na (-0.55) AP (0.84)* ACP (0.63)* Cl (-0.53) K (0.37) Na (-0.44) AP (0.86)* ACP (0.56)* Cl (-0.49) Na (-0.45) Total nr of sperm AP (0.63)* ACP (0.38) Ca (-0.35) Cl (-0.50) Na (-0.44) AP (0.61)* ACP (0.52) Pi (0.36)* AP (0.36) PGE2 (-0.42) Cl (-0.43) Na (-0.38)* K (-0.36) Pi (0.39) - - - - - Pi (0.40) - - Progressive motility Average path velocity Velocity, curved line Velocity, straight line Straightness Linearity DIFF in DFI LOW ( ) SP1 SP2 Total motility before storage Progressive motility before storage Morphologically normal sperm - 53 - 5.3. Proteins in seminal plasma fractions and correlations with sperm concentration and motility (III) 5.3.1. Differences in protein levels between stallions and seminal plasma fractions (III) In Experiment C (III: Trial 1), the proteins HSP-1, HSP-2, and HSP-4 (at <30 kD) were detected in the RP-HPLC and N-terminal sequencing of SP samples of all of the stallions. A <10-kD protein (probably acrosine inhibitor) and prostate specific antigen (PSA), or kallikrein, at 26 kD were also found in all stallions. HSP-1 was the major protein in all fractions, all stallions, and all ejaculates. The relative amounts of proteins are expressed as the area-under-the-curve (AUC) of the chromatograms. The chromatogram of ejaculate 1 of stallion 3 is presented in Fig. 8. No significant differences emerged between fractions or stallions in the number of peaks in RP-HPLC. The number of peaks varied from 4 to 8 in each sample; 8 peaks were found most frequently, in 38% of all samples. The peak containing HSP-4 followed HSP-1 and HSP-2, and it was one of the three highest peaks in all of the samples. This peak is likely to contain both HSP-3 and HSP-4, as HSP-3 is N-terminally blocked and not detectable with the N-terminal sequencing used in this experiment, and thus, this peak will be termed HSP-3/4. In mass spectrometry, HSP-1, HSP-2, HSP-3, HSP-4, PSA and the <10-kD protein were detected in all ejaculate fractions. No significant differences were present between fractions in the AUC values of HSP-1, HSP-2 or HSP-3/4. The AUC values of HSP-2 and HSP-3/4 were significantly different between stallions only in LOW. In Experiment D (III: Trial 2), the SP samples contained 6–12 protein bands. The amount of proteins at 60-70 kD was greater in LOW than in HIGH and WE, but no other significant differences existed between ejaculate fractions in the amount of other proteins. 54 Fig. 8. A RP-HPLC chromatogram, showing the fraction-specific protein peaks (cup 1 = HIGH, cup 3 = LOW). Peaks containing HSP-1, HSP-2 and HSP-4 are marked with respective N-terminal amino acid sequences. 55 5.4. The association between seminal plasma components and semen quality, sperm longevity, and fertility (III, IV) Descriptive data on fertility and the number of stallions and mares in Experiment E (IV) are provided in Table 8. There were no significant differences in pregnancy or foaling rates between breeding methods (insemination with fresh or transported semen). Table 8. Mean (± SE) values of pregnancy rate/season, foaling rate, number of cycles/pregnancy and first cycle pregnancy rate for all matings: inseminations with fresh semen, transported semen, and including natural breeding in Experiment E (IV). Stallions serving 20 mares All Fresh Transported inseminations semen semen N (stallions) N (mares) N (mares/stallion, min-max) N (inseminated cycles) 1st cycle pregnancy rate (%) Pregnancy rate/season (%) Foaling rate (%) Number of cycles/pregnancy 16 937 20-150 1538 51.6 ± 3.4a 80.0 ± 2.4a 70.2 ± 2.4a 2.15 ± 0.1a 7 281 20-69 663 60.5 ± 7.6a 83.0 ± 4.1a 73.4 ± 4.3a 1.98 ± 0.3a 9 483 20-113 853 50.1 ± 5.3a 74.2 ± 6.5a 65.2 ± 6.3a 2.15 ± 0.2a a,b Different letters indicate a significant difference within a row. In addition to the correlations listed in Table 7, the correlation between normal morphology and DFI before storage was found to be negative (-0.63, p = 0.000) in Experiment E (IV). In Experiment C (III: Trial 1), the AUC values of HSP-1, HSP-2 and HSP-3/4 were significantly and positively correlated with sperm concentration, but not with TMOT, PMOT or VAP. In Experiment D (III: Trial 2), proteins at the 70- to 90-kD peak were negatively correlated with the sperm concentration; otherwise, no significant correlations between protein levels and the sperm concentration could be detected. In the LOW fraction in Experiment E (IV), a significant association of K (coefficient of determination (R2) = 0.26) with the DIFF in TMOT emerged in the linear regression analysis, whereas none of the SP components affected the mean DIFF in any of the other motility parameters or in DFI in any of the fractions. The concentration of K was not significantly correlated with any of the sperm motility parameters in any of the ejaculate fractions. The sperm concentration was associated with the levels of AP in HIGH (R2 = 0.47), and both AP and ACP in LOW (AP: R2 = 0.50, ACP: R2 = 0.61) and WE (AP: R2 = 0.68, ACP: R2 = 0.74). The total number of sperm was associated with AP in HIGH (R2 = 0.28) and LOW (R2 = 0.63), Pi in LOW (R2 = 0.55) and Na in WE (R2 = 0.19) (Table 7). 56 None of the studied components of SP were significantly correlated with the first cycle pregnancy rate. In summary, the levels of AP, ACP and the AUC values of HSP-1, HSP-2 and HSP-3/4 are associated with sperm concentration. In the LOW fraction, the levels of K are associated with the DIFF in TMOT, while none of the components showed any correlation with the first cycle pregnancy rate. 57 6. Discussion 6.1. Effects of the removal of seminal plasma on sperm longevity (I, IV) According to our results, the removal of SP causes a decrease in several motility parameters, but also a concurrent and perhaps paradoxical decrease in DFI and a decrease in STR and LIN. Previous reports have suggested an association between DNA integrity and fertility (Love and Kenney 1998, Morrell et al. 2008). Experiments on SP removal have yielded contradicting results, as summarized in the literature review of this thesis. Several studies have shown a beneficial effect of SP removal on sperm motility (Jasko et al. 1991, Pruitt et al. 1993, Braun et al. 1994, Todd et al. 2001, Alghamdi et al. 2002), but as stated earlier, the effects of SP removal are dependent on the choice of extender, which will inevitably vary in osmolarity, pH and composition (Padilla and Foote 1991, Bedford et al. 1995, Akcay et al. 2006). Several factors, such as choice of extender, variation in the composition of SP between stallions and ejaculations, variation in semen quality between stallions, and also the interactions between all these, are likely to have an effect on the results of storage studies. Sperm concentration does not seem to have an effect on sperm survival when the effect of the proportion of SP is eliminated (Voge et al. 2010). Recently, Barrier-Battut et al. (2010) demonstrated that sperm motility and acrosome integrity without ionophore challenge were unaffected by SP removal after 48 h of cooled storage, but PMI was greater, and the responsiveness to induction of acrosome exocytosis was reduced, in samples stored without SP. The proportion of SP varied between 4.5% and 33% in the mentioned study, and the effects of SP did not appear to be dose-dependent. The intention in both Experiment A and E was not to remove SP completely, but to leave approximately 5% of SP in the samples, because sperm motility after complete removal of SP has been reported to be affected by the type of extender (Padilla and Foote 1991, Webb and Arns 1995, Akcay et al. 2006). A plausible explanation for the decrease in motility in the samples stored without SP in Experiments A and E is that the proportion of SP was too low (<5%), as the final volume of SP could not be accurately measured at the time of the final extension. The higher rate of DNA integrity in samples stored without SP seen in Experiment E (IV), despite the maintenance of sperm motility, is in accordance with earlier work by Love et al. (2005). The conflicting effects on sperm motility and DNA integrity, and especially the increase in the proportion of sperm with high VCL and low STR and LIN, raise the suspicion that hyperactivation and premature capacitation of sperm could have affected the mean motility values in these studies. In humans, heat induction of hypermotility has been proposed as a means of improving total sperm motility, and also pregnancy rates (Kücük et al. 2008). In stallion semen incubated in a capacitating bicarbonate-containing media for 5 h at 37 °C, 5.3 ± 2.4% of motile spermatozoa were hyperactive, when sperm with a VCL of 180 µm/s and an amplitude of lateral head displacement of 12 µm were considered hyperactive. This was a smaller proportion than the proportion of capacitated sperm (Rathi 58 et al. 2001), indicating that hyperactivity may not depict the capacitation status very clearly. In Experiment E (IV), the proportion of hyperactive sperm (VCL 180 µm/s) in SP1 was 7.5% in HIGH, 26.5% in LOW and 22.0% in WE. In SP0 the corresponding values were 0%, 8.3% and 9.8%. The higher proportion of SP may thus have inhibited the hyperactivation of sperm, but the capacitation status was not evaluated in this study. As SP is known to contain decapacitation factors (Suzuki et al. 2002), it could prevent premature capacitation, and it has been reported that the removal of SP increases the capacitationassociated Ca++ influx into sperm cells (Magistrini et al. 1997). The hyperactivation of stallion spermatozoa requires further investigation, but at this point it is useful to recognize the fact that sperm motility does not always reliably portray the fertilizing capacity of spermatozoa. Spermatozoa need numerous functional attributes to fertilize an ovum, and it would be useful to include more semen quality parameters in studies evaluating the effects of SP removal on sperm survival. Further studies in this area using modern applications are certainly warranted, as many studies have mostly focused on sperm motility, which is not a reliable predictor of fertility (Kuisma et al. 2006, Varner et al. 2008). In Experiment A (I), the samples stored with SP were not centrifuged, and sperm longevity in the samples stored without SP could have been harmed by centrifugation. However, a similar effect of the removal of SP was again seen in Experiment E (IV), where the samples in both storage groups (SP1 and SP0) were centrifuged, and the number of stallions was greater. Other studies have shown that centrifugation at a similar force (500 g) does not have an adverse effect on sperm survival during storage (Jasko et al. 1991, Barrier-Battut et al. 2010). The low number of stallions in Experiments A–D is one of the main challenges in interpreting the results of these experiments. Some of the stallions in Experiments A-C were older stallions with suboptimal semen quality, and thus the true effect of SP on sperm longevity may not have been detected in those experiments. Considering the evident stallion variation, the characteristics of an individual stallion could easily have affected the results. As shown in Figures 4 and 5, the effects of SP on motility could be exactly the opposite in two randomly selected stallions. Furthermore, samples were collected outside of the breeding season. Season does affect semen quality parameters, such as semen volume, sperm concentration, total sperm count, motility of fresh semen (Janett et al. 2003), and freezability (Janett et al. 2003, Wrench et al. 2010), but on the other hand, the current experiments compared different treatments on the same samples. The results of the larger experiment (Experiment E) were well in line with the other experiments (A–D). Because of the inherent variability between stallions, it may be useful to modify the semen processing procedures for stallions producing semen with poor tolerance of storage or with SP “toxicity”, as has previously been recommended (Brinsko and Varner 1992, Brinsko et al. 2000, Love et al. 2005). 59 6.2. Sperm survival during storage in sperm-rich and sperm-poor fractions (I, IV) Based on earlier reports (Webb et al. 1990, Varner et al. 1987, Sieme et al. 2004), it was hypothesized that the sperm-rich fractions would maintain sperm longevity better than whole ejaculates. The results of Experiments A and E (I, IV) indicate that sperm motility after cooled storage is similar in sperm-rich and sperm-poor fractions, and that the lowest rate of DNA damage was found in the sperm-rich fractions after the removal of SP. When SP was present during storage, the amount of DNA damage was more equal in the ejaculate fractions, but higher than in the sperm-rich fraction stored without SP. Sieme et al. (2004) reported that centrifuged sperm-rich fractions had higher sperm motility and PMI than non-centrifuged sperm-rich fractions and centrifuged whole ejaculates. Lower plasma membrane integrity and PMOT has been reported in sperm stored in SP from the sperm-rich fractions compared with that stored in SP from the sperm-poor fractions, and it seems that the sperm-rich fraction may benefit more from SP removal than the sperm-poor fraction, and that SP from the sperm-rich fractions may be more harmful for sperm survival during storage (Akcay et al. 2006). The presence of pre-sperm fluid in the sperm-rich fractions was mentioned by Akcay et al. (2006) as an explanation for the decrease in sperm survival in the sperm-rich fraction, and this could also have been the case in Experiment E. It would be interesting to examine the effect of pre-sperm fluid on DNA integrity and pregnancy rates. If there is a need to try to improve sperm longevity in an individual stallion, the collection of the sperm-rich fraction separately followed by the removal of most of the SP could be recommended based on these results. Avoiding the inclusion of pre-sperm fluid in transported insemination doses may also be useful, but definitive evidence of any harmful effects of pre-sperm fluid are lacking. It is worth noting that the fractioning of the ejaculates differed between the experiments presented in this thesis, and also between the works presented by other research groups, making the comparison of the sometimes conflicting results difficult. Ejaculates of stallions producing ejaculates with low volume and high sperm concentrations were often difficult to fractionate, as the semen jets were nonexistent or virtually impossible to separate. In the studies presented in this thesis, this type of ejaculate usually had to be examined as a WE sample only. Ejaculates containing large volumes of SP were simple to collect as fractions, as the semen jets were easily distinguishable. The difficulties in fractioning decreased the number and volume of samples for analyses. The results of the various published storage studies are affected by the method of fractioning, but also by other factors, such as the length of storage, the type of extender, the proportion of SP in the stored samples, and which semen quality parameters have been selected to depict sperm longevity. 60 6.3. Levels of seminal plasma components in sperm-rich and sperm-poor fractions (II, III, IV) In Experiment D, the highest levels of Na were found in pre-sperm fluid, and thus the presence of some pre-sperm fluid in the sperm-rich fraction in Experiments A and E may have raised the concentration of Na in this fraction. The high levels of Na and Cl in presperm fluid were demonstrated in the early studies by Mann et al. (1956, 1963). The electrolyte composition in SP and extenders has been suggested to be of importance to sperm survival during storage (Padilla and Foote 1991). The effects of Na and pre-sperm fluid are further discussed in Section 6.4. The association between sperm concentration and AP has been documented in earlier reports (Turner et al. 2003, Pesch et al. 2006), and was confirmed in the experiments presented here. The levels of AP differed somewhat in magnitude between Experiments A, D and E (II, IV), which is probably due to the small number of stallions in Experiment A (II), allowing individual variation to affect the mean values. The samples in Experiment D were collected in February, as opposed to the samples in Experiment E that were collected in the end of the breeding season. Variation due to season has been documented for semen quality parameters, such as semen volume, sperm concentration, and total sperm count (Heckenbichler et al. 2010), and for the composition of SP (Abou-Ahmed et al. 1993, Gebauer et al. 1976). The method of AP analysis was the same in all studies, and the same person performed all of the analyses. Characteristic of the sperm-rich fractions were the high levels of AP, ACP and BG, whereas higher levels of Ca and Mg were seen in the sperm-poor fractions. As AP is mainly derived from the testes and epididymis (Turner and McDonnell 2003), the high levels of AP in the sperm-rich fractions suggests that most of the testicular and epididymal SP components are released in the first ejaculatory jets. The seminal vesicles are a likely source of Ca and Mg, as the last ejaculatory jets mainly contain vesicular secretions (Mann et al. 1956). The major SP proteins were detected in all ejaculate fractions, and there were no differences between fractions in the number of peaks found in the chromatograms, or in the relative amount of the studied proteins in the RP-HPLC. The total protein level is lower in pre-sperm fluid than in the other fractions (Koskinen et al. 2002). Some fraction variation was detected in the SDS-PAGE, but based on the methods used, it is not possible determine which particular protein is involved. The difference in the origin of SP in the sequentially formed fractions may have caused the variation in protein composition seen in the SDS-PAGE, where the 60- to 70-kD proteins were more abundant in the sperm-poor fraction. The proteins present in this electrophoretic band are likely to mainly originate from the seminal vesicles, as the last fractions of the ejaculate are predominantly seminal vesicle fluid (Weber and Woods 1993, Magistrini et al. 2000). It is difficult to draw any 61 conclusions about the origin of specific proteins or protein groups in the first ejaculate fractions, as they are composed of fluids from several glands. However, HSP-1 and HSP-2 have been shown to mainly be produced in the ampulla, and a smaller portion in the cauda epididymis (Ekhlasi-Hundrieser et al. 2005). Because the subsequent SP fractions come in contact with each other at least indirectly during ejaculation, all of the proteins found in the RP-HPLC were detected in all fractions. 6.4. Contents of seminal plasma in relation to sperm survival during storage and fertility (III, IV) An association between K and the difference in TMOT between samples stored with SP and samples stored without SP emerged in Experiment E (IV). With decreasing concentrations of K in SP, the presence of SP leads to an increase in TMOT, and the removal of SP with a lower K content will reduce TMOT values. In the presence of higher concentrations of K in the LOW fraction, TMOT in SP0 and SP1 were more equal. In other words, it is plausible that the presence of SP with a low K content could be less harmful for sperm motility than SP with a higher K content. The levels of K have not been shown to be correlated with semen volume, sperm concentration, motility, viability or morphological abnormalities in stallion semen (Pesch et al. 2006), or with sperm motility in men (Rossato et al. 2002), and this is well in agreement with the present results. The addition of Na and K to storage media has been shown to induce lipid peroxidation of sperm membranes (Alvarez and Storey 1982). Padilla and Foote (1991) discussed K levels as a potential factor affecting the maintenance of motility during cooled storage. They reported that the levels of K were 26 mM in SP, 1 mM in skim milk extender, and 10 mM in skim milk extender supplemented with a modified Tyrode’s solution (KMT). Thus, the K content in skim milk extender is lower than in SP or KMT. The pH in a Kenney-type skim milk extender has also been shown to be significantly lower, and the osmolarity higher, than in KMT and in SP from the sperm-rich and sperm-poor fractions (Padilla and Foote 1991, Akcay et al. 2006). A traditional Kenney’s skim milk extender is not the ideal storage medium after complete (or nearly complete) removal of SP (Padilla and Foote 1991, Akcay et al. 2006). When non-centrifuged samples containing 20% SP were studied, the samples stored in skim milk extender maintained motility better than samples stored in the modified extender with higher potassium content. In centrifuged samples, the samples stored in the high-potassium extender maintained higher motility than samples stored in skim milk extender (Padilla and Foote 1991). Similar results have been reported by Webb and Arns (1995). According to Rigby et al. (2001), KMT was a superior storage medium compared to Kenney’s skim milk extender, irrespective of the presence of SP. All in all, adjusting the ion content of cooled semen by adding extenders with lower K content than in SP is likely to have a beneficial effect on sperm motility. Further investigations on the ion content of SP and its effects on sperm survival are needed, however, before definitive suggestions can be made on the ideal ionic composition of semen extenders. 62 Pre-sperm fluid contains higher levels of both Na and Cl than the other parts of the ejaculate, but pre-sperm fluid did not seem to have any radical negative or positive effects on motility or membrane integrity. In Experiment A, spermatozoa from the sperm-rich fraction were stored cooled for 24 h in pre-sperm fluid and skim milk extender. Because of difficulties collecting adequate amounts of pre-sperm fluid separately, the number of samples was low (n = 6) and the pre-sperm fluid samples were not included in the statistical analysis. Based on the descriptive statistics, the motility parameters TMOT, PMOT and RAP were numerically only slightly lower in pre-sperm fluid than in the sperm-rich fractions, but higher than in the sperm-poor fractions. The PMI was slightly higher in pre-sperm fluid compared to both sperm-rich and sperm-poor fractions. In Experiment E, the sperm-rich fraction benefited more from SP removal than the spermpoor fraction, as DNA integrity was maintained best in the sperm-rich fraction without SP, and while this could be associated with the presence of pre-sperm fluid in the first jets of the ejaculate, the direct effects of pre-sperm fluid on DNA integrity are not known. Further research on the effects of pre-sperm fluid on sperm survival is needed. The enzymes AP and ACP were positively correlated to sperm concentration in the present study, and have earlier been shown to be negatively correlated with the semen volume (Pesch et al. 2006). The positive correlation between AP and both sperm concentration and the total number of sperm is well in accordance with previous work (Turner and McDonnell 2003). None of the measured components of SP served as reliable predictors of stallion fertility. However, the fertility assessment should be interpreted with caution, as the number of both stallions and mares was low. Experiment E aimed at scanning a larger population of stallions for a SP composition profile compatible with good fertility, to try to reveal which SP components, if any, would have a clear association with fertility. The draw back is the use of single ejaculates. The analyzed ejaculates were not used for inseminations; instead, the pregnancy rates were calculated based on data from the whole season. Because semen quality can change over time, and many stallion-independent factors influence pregnancy rates, it is challenging to interpret the significance of the effects of SP components on fertility. When only those stallions who served at least 20 mares were included in the statistical analysis, the differences in pregnancy and foaling rates between inseminations with fresh or transported semen were non-significant. As these stallions were in higher demand and have higher breeding fees, it is likely that the more intensive veterinary care and breeding management gave better results also for inseminations with transported semen. When all stallions were included, the first cycle pregnancy rate was higher when fresh semen was used, compared to inseminations with cooled semen. Recently, Brinkerhoff et al. (2010) reported that a mare bred with fresh semen had a 2.5 times higher probability of becoming pregnant than a mare bred with cooled, transported semen at a site other than the stud farm. 63 When high quality semen was used, sperm quality and the pre-breeding status of the mare (i.e. barren, foaling or maiden) did not influence fertility, but mare age and the breeding method were critical factors affecting fertility. Successful management at the receiving station is therefore essential for achieving high pregnancy rates. It has also been reported that inseminations with transported semen at artificial insemination centers result in higher pregnancy rates than inseminations at the breeder’s own facilities (Rota et al. 2004), and if the semen quality of transported semen used for insemination at the breeder’s facilities is poor, the pregnancy rate could be expected to decrease even further. As the use of cooled transported semen in Finland has increased, the foaling rates have decreased (Katila et al. 2010b). Stud farms need to ensure that the sperm longevity in their transported insemination doses is optimized. This can be achieved by correct semen handling procedures, such as avoiding cold shock and prolonged exposure to light (reviewed by Katila 1997), applying individual modification of semen processing and storage conditions, such as removing SP from semen before cooling and transport (Brinsko and Varner 1992, Brinsko et al 2000, Love et al. 2005), and by selecting breeding methods suitable for individual stallions, so that stallions producing semen with a poor tolerance of storage are only recommended for insemination with fresh semen. 64 7. Conclusions 1. There were no differences in the maintenance of sperm motility between the sperm-rich and sperm-poor fractions and whole ejaculates during cooled storage, irrespective of the presence of seminal plasma (I, IV). 2. The removal of seminal plasma reduced sperm motility (I, IV) but improved DNA integrity during cooled storage compared with samples containing seminal plasma (IV), suggesting that the presence of seminal plasma may increase the amount of DNA damage in stored spermatozoa. However, marked between-stallion variation exists in the effects of seminal plasma on sperm longevity, and some stallions may need individual modifications of the semen processing procedures. 3. The lowest rate of DNA damage was found in the sperm-rich fractions stored without seminal plasma (IV), indicating that spermatozoa in the sperm-rich fraction may benefit more from the removal of seminal plasma than those in the sperm-poor fraction. 4. Post-thaw sperm motility was highest in the sperm-rich fraction (I), suggesting that the collection of fractionated ejaculates could be beneficial when freezing semen. 5. The sperm-rich and sperm-poor fractions differ in composition. The sperm-rich fractions contained the highest levels of the enzymes alkaline phosphatase, acid phosphatase, and glucuronidase. The sperm-poor fractions contained the highest concentrations of calcium and magnesium. The highest levels of sodium and chloride were found in the pre-sperm fluid (II, IV). 6. The major proteins of stallion seminal plasma, HSP-1 and HSP-2, were found in all fractions and were positively correlated with sperm concentration (III). 7. The level of potassium was higher in the sperm-poor fraction than in the sperm-rich fraction. Lower concentrations of potassium in the sperm-poor fraction led to higher total motility after storage when seminal plasma was present during storage, while the removal of seminal plasma with lower potassium content reduced motility. The level of potassium may be of importance when choosing a suitable extender and semen processing regimen for subfertile stallions (IV). 65 Acknowledgments The research presented in this thesis was carried out at the Department of Production Animal Medicine at the Faculty of Veterinary Medicine (University of Helsinki). Most of the work was funded by the Mercedes Zachariassen foundation and the Foundation for Veterinary Medicine in Finland (The Finnish Veterinary Association). I would like to thank my mentor and supervisor Professor Terttu Katila for all her hard work in guiding me through this project, for her wisdom and integrity, and for teaching me about science and equine reproduction. I am truly grateful to Professor Katila and to the Department of Production Animal Medicine for giving me the opportunity to participate in this research. I would also like to thank my other supervisors for their input in this project; Professor Magnus Andersson at the Department of Production Animal Medicine, for always being so funny and entertaining, but especially for his insights in the field of andrology, and for his never-ending encouragement. I am also grateful and honored to have had Professor Heriberto Rodriguez-Martinez (Linköping University) as one of my supervisors. His scientific and critical approach in teaching has instigated much improvement in my research. I would like to thank my two pre-examiners Michèle Magistrini and Anne-Marie Dalin for the time and effort they put into evaluating my work and providing constructive criticism and helpful comments. Professor Harald Sieme is thanked for agreeing to act as my opponent. I am grateful to everyone who contributed to this work. Satu Sankari at the Central Laboratory of the Faculty of Veterinary Medicine at the University of Helsinki spent a lot of time on the biochemical analyses and discussing the methods and results with me. Maria Rivera del Alamo at the University of Barcelona made a huge effort in getting our samples from Valencia to Barcelona and doing the gel electrophoresis. Juan Calvete and his colleagues at the Institute of Biomedicine in Valencia did all the advanced proteomics work presented in this thesis, explained it to me (again and again), and helped in editing the protein paper (publication III). Anders Johannisson and Hans Kindahl at SLU in Uppsala kindly contributed to the analyses in Experiment E by performing the SCSA and PGE2 assays. Tero Wahlberg at the University of Turku provided expert assistance with the statistical analyses in Experiment E. I am greatly indebted to Tiina “Skipper” Reilas at MTT Agrifood Research Finland, for teaching me mare gynecology, for always helping me when I am lost, for diligently and thoroughly going through version after version of maturing manuscripts, and for being a good friend. 66 This research could not have been done without the help of the wonderful staff at MTT Agrifood Research Finland; all of the barn managers and Marja Rauvola at the MTT lab assisted in this study and have taught me a lot. I am also grateful to all of the cooperative and helpful stud farms, stud farm veterinarians and horse owners who participated in Experiment E: MTT Agrifood Research Finland, Metsäpirtti, Kallelan oriasema, Aria Stable, Skyhorses, Sillanpään oriasema, Kylämäen Hevostila, Keskisen oriasema, and RokTalli. Merja-Liisa Toivonen and Metsäpirtti are especially acknowledged for all the friendly help and cooperation during these years. I would also like to acknowledge my dear friends, the horsey girls from the days of yore, for supporting me during this time. A special thank you goes out to all of my dear friends and colleagues at the Production Animal Hospital and the Department of Production Animal Medicine; working with you is an inspiration. The friendly and fun atmosphere in Saari is one of a kind. All of my colleagues who have given me strength and belief, and shared these years with me; thank you, you know who you are. Last, but definitely not least, I would like to thank my family; my husband Kim for putting up with this sometimes grumpy “testicle doctor”, as someone so eloquently once called me, and for everything we’ve shared together. It’s always good to come home. My parents, for not allowing me to quit school and be a groom, and for always encouraging me and believing in all the strange things I think of doing. My brother, for not bullying me anymore, and for being my idol. And many thanks to my beloved daughter Kira, who I never dared to think we’d be lucky enough to have. Maria Kareskoski 67 References Abdel-Rahman HA, El-Belely MS, Al-Qarawi AA, El-Mougy SA, 2000: The relationship between semen quality and mineral composition of semen in various ram breeds. Small. Rum. Res. 38 45-49. Abou-Ahmed MM, El-Belely MS, Ismail ST, El-Baghdad YRM, Hemeida NA, 1993: Influence of age and season on certain biochemical constituents of seminal plasma of Arabian horses. Anim. Reprod. Sci. 32 237-244. Adamopoulos DA, Deliyiannis V, 1983: Seminal plasma magnesium, calcium and inorganic phosphate concentration in normozoospermic and subfertile men. Andrologia 15 648-654. Akcay E, Reilas T, Andersson M, Katila T, 2006: Effect of seminal plasma fractions on sperm survival after cooled storage. J. Vet. Med. A. 53 481-485. Alghamdi AS, Troedsson MH, Xue JL, Crabo BG, 2002: Effect of seminal plasma concentration and various extenders on postthaw motility and glass wool-Sephadex filtration of cryopreserved stallion semen. Am. J. Vet. Res. 63 880-885. Alvarez JG, Storey BT, 1982: Spontaneous lipid peroxidation in rabbit epididymal spermatozoa: its effect on sperm motility. Biol Reprod. 27 1102-8. Amann RP, Cristanelli MJ, Squires EL, 1987: Proteins in stallion seminal plasma. J. Reprod. Fert. Suppl. 35 113-120. Archbald L, Gronwall RR, Pritchard EL, Tran T, 1990: Acrosome reaction and concentration of prostaglandin E2 in semen of rams treated with flunixin meglumine (Banamine). Theriogenology 33 373-83. Arienti G, Carlini E, De Cosmo AM, Di Profio P, Palmerini CA, 1998: Prostasome-like particles in stallion semen. Biol. Reprod. 59 309-313. Asari M, Sasaki K, Miura K, Ichihara N, Nishita T, 1996: Immunohistolocalization of the carbonic anhydrase isoenzymes (CA-I, CA-II and CA-III) in the reproductive tract of male horses. Am. J. Vet. Res. 57 439-443. Aumüller G, Seitz J, Lilja H, Abrahamsson P-A, von der Kammer H, Scheit K-H, 1990: Species- and organ-specificity of secretory proteins derived from human prostate and seminal vesicles. Prostate 17 31-40. Aurich JE, Kühne A, Hoppe H, Aurich C, 1996: Seminal plasma affects membrane integrity and motility of equine spermatozoa after cryopreservation. Theriogenology 46 791-797. 68 Baker MA, Hetherington L, Ecroyd H, Roman SD, Aitken RJ, 2004: Analysis of the mechanisms by which calcium negatively regulates the tyrosine phosphorylation cascade associated with sperm capacitation. J. Cell. Sci. 117 211-222. Ball BA, Gravance CG, Medina V, Baumber J, Liu IKM, 2000: Catalase activity in equine semen. Am. J. Vet. Res. 61 1026-1030. Ball BA, Vo AT, Baumber J, 2001: Generation of reactive oxygen species by equine spermatozoa. Am. J. Vet. Res. 62 508-515. Ball BA, Gravance CG, Wessel MT, Sabeur K, 2003: Activity of angiotensin-converting enzyme (ACE) in reproductive tissues of the stallion and effects of angiotensin II on sperm motility. Theriogenology 59 901-914. Barbieri MA, Sosa MA, Couso R, Ielpi L, Merello S, Tonn CE, Bertini F, 1994: Affinity sites for N-acetyl- -D-glucosaminidase on the surface of rat epididymal spermatozoa. Int. J. Androl. 17 43-49. Barbieri MA, Veisaga ML, Paolicchi F, Fornes MW, Sosa MA, Mayorga LS, BustosObregón E, Bertini F, 1996: Affinity sites for -glucuronidase on the surface of human spermatozoa. Andrologia 28 327-333. Baronos S, 1971: Seminal carbohydrate in boar and stallion. J. Reprod. Fert. 24 303-305. Barrier-Battut I, Delajarraud H, Legrand E, Bruyas J-F, Fiéni F, Tainturier D, Thorin C, Pouliquen H, 2002: Calcium, magnesium, copper, and zinc in seminal plasma of fertile stallions, and their relationship with semen freezability. Theriogenology 58 229-232. Barrier-Battut I, Dacheux JL, Gatti JL, Rouviere P, Stanciu C, Dacheux F, Vidament M, 2005: Seminal plasma proteins and semen characteristics in relation with fertility in the stallion. Anim. Reprod. Sci. 89 255-258. Barrier-Battut I, Bonnet C, Giraudo A, Dubois C, Caillaud M, Vidament M, 2010: Removal of seminal plasma by centrifugation, before cooled storage, enhances membrane stability of stallion spermatozoa. Anim. Reprod. Sci 121 188-190. Bauer PJ, 1981: Affinity and stoichiometry of calcium binding by arsenazo III. Anal. Biochem. 110 61 -72. Baumber J, Ball BA, Gravance CG, Medina V, Davies-Morel MC, 2000: The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 21 895902. 69 Baumber J, Vo A, Sabeur K. Ball BA, 2002: Generation of reactive oxygen species by equine neutrophils and their effect on motility of equine spermatozoa. Theriogenology 57 1025-1033. Baumber J, Sabeur K, Vo A, Ball BA, 2003: Reactive oxygen species promote tyrosine phosphorylation and capacitation in equine spermatozoa. Theriogenology 60 1239-1247. Baumber J, Ball BA, 2005: Determination of glutathione peroxidase and superoxide dismutase-like activities in equine spermatozoa, seminal plasma, and reproductive tissues. Am. J. Vet. Res. 66 1415-1419. Baumber J, Ball BA, Linfor JJ, 2005: Assessment of the cryopreservation of equine spermatozoa in the presence of enzyme scavengers and antioxidants. Am. J. Vet. Res. 66 772–779. Baumgart E, Lenk SV, Loening SA, Jung K, 2002: Quantitative differences in matrix metalloproteinase (MMP)-2, but not in MMP-9, tissue inhibitor of metalloproteinase (TIMP)-1 or TIMP-2, in seminal plasma of normozoospermic and azoospermic patients. Hum. Reprod. 17 2919-2923. Bedford SJ, Graham JK, Amann RP, Squires EL, Pickett BW, 1995: Use of two freezing extenders to cool stallion spermatozoa to 5°C with or without seminal plasma. Theriogenology 43 939-953. Beyler SA, Zaneveld LJD, 1982: The male accessory sex glands. In: Biochemistry of mammalian reproduction. Eds: Zaneveld LJD, Chatterton RT. John Wiley & Sons, NY, USA. pp. 65-88. Björndahl L, Kvist U, 2003: Sequence of ejaculation affects the spermatozoon as a carrier and its message. Reprod. Biomed. Online 70 440-448. Blanchard TL, Varner DD, Schumacher J, Love CC, Brinsko SP, Rigby SL, 2003: Semen preservation. In: Manual of equine reproduction. 2nd edition. Mosby, St. Louis, USA. pp. 165-176. Bollwein H, Sowade C, Stolla R, 2001: The effect of semen extender, seminal plasma and raw semen on uterine and ovarian blood flow ipsi- and contralateral to the preovulatory follicle in mares. Anim. Reprod. Sci. 68 346. Brandelli A, Miranda PV, Tezon JG, 1994: Participation of glycosylated residues in the human sperm acrosome reaction: possible role of N-acetylglucosaminidase. Biochim. Biophys. Acta 17 299-304. Brandon CI, Heusner GL, Caudle AB, Fayrer-Hosken, 1999: Two-dimensional polyacrylamide gel electrophoresis of equine seminal plasma proteins and their correlation with fertility. Theriogenology 52 863-873. 70 Braun J, Sakai M, Hochi S, Oguri N, 1994: Preservation of ejaculated and epididymal stallion spermatozoa by cooling and freezing. Theriogenology 41 809-818. Braun J, Muto Y, Sato K, Schallenberger E, 1996: Der Einfluss von Jahreszeit und sexueller Beanspruchung auf die Konzentration von Testosteron und Östradiol-17 im Seminalplasma beim Hengst (Influence of season and ejaculation frequency upon concentrations of testosterone and estradiol-17 in seminal plasma of stallions). Tierärztl. Prax. 24 577-580. Brinkerhoff JM, Love CC, Thompson JA, Blodgett G, Teague SR, Varner DD, 2010: Influence of mare age, pre-breeding mare status, breeding method, and stallion on first cycle pregnancy rates on a large commercial breeding farm. Anim. Reprod. Sci. 121S 159. Brinsko SP, Varner DD, 1992: Artificial insemination and preservation of semen. Vet. Clin. N. Am. Equine Pract. 8 205-218. Brinsko SP, Crockett EC, Squires EL, 2000: Effect of centrifugation and partial removal of seminal plasma on equine spermatozoal motility after cooling and storage. Theriogenology 54 129-136. Buchman-Shaked O, Kraiem Z, Gonen Y, Goldman S, 2002: Presence of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinase in human sperm. J. Androl. 23 702-708. Caballero I, Vazquez JM, Gil MA, Calvete JJ, Roca J, Sanz L, Parrilla I, Garcia E, Rodriguez-Martinez H, Martinez EA, 2004: Does seminal plasma PSP-1/PSP-II spermadhesin modulate the ability of boar spermatozoa to penetrate homologous oocytes in vitro? J. Androl. 25 1004-1012. Calvete JJ, Nessau S, Mann K, Sanz L, Sieme H, Klug E, Töpfer-Petersen E, 1994: Isolation and Biochemical characterization of stallion seminal-plasma proteins. Reprod. Domest. Anim. 29 411-426. Camejo MI, 2003: Relation between immunosuppressive PGE(2) and IL-10 to proinflammatory IL-6 in seminal plasma of infertile and fertile men. Arch. Androl. 49 111116. Cancel AM, Chapman DA, Killian GJ, 1997: Osteopontin is the 55-kilodalton fertilityassociated protein in Holstein bull seminal plasma. Biol. Reprod. 57 1293-1301. Carver DA, Ball BA, 2002: Lipase activity in stallion seminal plasma and the effect of lipase on stallion spermatozoa during storage at 5 C. Theriogenology 58 1587-1595. 71 Centurión F, Vazquez JM, Calvete JJ, Roca J, Sanz L, Parrilla J, Garcia EM, Martinez EA, 2003: Influence of porcine spermadhesins on the susceptibility of boar spermatozoa to high dilution. Biol. Reprod. 69 640-646. Claus R, Dimmick MA, Gimenez T, Hudson LW, 1992: Estrogens and prostaglandin F2 in the semen and blood plasma of stallions Theriogenology 38 687-693. Cremades T, Carvajal G, Hernandez M, Calvete JJ, Sanz L, Vazquez JM, Martinez EA, Roca J, 2004: Freezing of boar semen is not affected by the addition of seminal plasma spermadhesins. Reprod. Domest. Anim. 39 269. Coleman JE, 1992: Structure and mechanism of alkaline phosphatase. Ann. Rev. Biophys. Biomol. Struct. 21 441-483. Corrales JJ, Burgo RM, Miralles JM, Villar EV, 2000: Abnormalities in sperm acid glycosidases from infertile men with idiopathic oligoasthenoteratozoospermia. Fertil. Steril. 73 470-478. Corrales JJ, Burgo RM, Galindo P, Muñoz-Barroso, Miralles JM, Villar EV, 2002: Abnormal expression of acid glycosidases in seminal plasma and spermatozoa from infertile men with varicocele. Reproduction 123 411-417. Daly JA, Ertigshausen G, 1972: Direct method for determining inorganic phosphate in serum with the "CentrifiChem". Clin. Chem. 18 263 -265. Danek J, 2001: Effect of dexamethasone treatment on the biochemical characteristics of the stallion semen. Bull. Vet. Inst. Pulawy 45 275-280. Denison FC, Grant VE, Calder AA, Kelly RW, 1999: Seminal plasma components stimulate interleukin-8 and interleukin-10 release. Mol. Hum. Reprod. 5 220-6. Dias AJ, Maia MS, Retamal CA, López ML, 2004: Identification and partial characterization of alpha-1,4-glucosidase activity in equine epididymal fluid. Theriogenology 61 1545-1558. Edorh AP, Tachev K, Hadou T, Gbeassor M, Sanni A, Creppy EE, LeFaou A, Rihn BH, 2003: Magnesium content in seminal fluid as an indicator of chronic prostatitis. Cell. Mol. Biol. 49 419-423. Einarsson S, Viring S, 1973: Distribution of frozen-thawed spermatozoa in the reproductive tract of gilts at different time intervals after insemination. J. Reprod. Fertil. 32 117-20. Ekhlasi-Hundrieser M, Schäfer B, Kirchhoff C, Hess O, Bellair S, Muller P, TöpferPetersen E, 2005: Structural and molecular characterization of equine sperm-binding fibronectin-II module proteins. Mol. Reprod. Dev. 70 45-57. 72 El-Hajj Ghaoui R, Thomson PC, Evans G, Maxwell WMC, 2004: Characterization and localization of membrane vesicles in ejaculate fractions from the ram, boar and stallion. Reprod. Dom. Anim. 39 173-180. Fouchécourt S, Métayer S, Locatelli A, Dacheux F, Dacheux J-L, 2000: Stallion epididymal fluid proteome: Qualitative and quantitative characterization; secretion and dynamic changes of major proteins. Biol. Reprod. 62 1790-1803. Frazer GS, Bucci DM, 1996: SDS-PAGE characterization of the proteins in equine seminal plasma. Theriogenology 46 579-591. Frenette G, Dube JY, Tremblay RR, 1986: Origin of alkaline phosphatase of canine seminal plasma. Arch. Androl. 16 235-241. Gebauer MR, Pickett BW, Faulkner LC, Remmenga EE, Berndtson WE, 1976: Reproductive physiology of the stallion. VII. Chemical characteristics of seminal plasma and spermatozoa. J. Anim. Sci. 43 626-632. Gerena RL, Irikura D, Urade Y, Eguchi N, Chapman DA, Killian GJ, 1998: Identification of a fertility-associated protein in bull seminal plasma as lipocalin type prostaglandin D synthase. Biol. Reprod. 58 826-833. Gindler EM, Heth DA, 1971: Colorimetric determination with bound “calmagite” of magnesium in human blood serum. Clin. Chem. 17 662. Greube A, Müller K, Töpfer-Petersen E, Herrmann A, Müller P, 2004: Interaction of fibronectin type II proteins with membranes: the stallion seminal plasma protein SP-1/2. Biochemistry 43 464-472. Griveau JF, Le Lannou D, 1997: Reactive oxygen species and human spermatozoa: physiology and pathology. Int. J. Androl. 20 61-69. Hamann H, Jude R, Sieme H, Mertens U, Töpfer-Petersen E, Distl O, Leeb T, 2007: A polymorphism within the equine CRISP3 gene is associated with stallion fertility in Hanoverian warmblood horses. Anim. Genet. 38 259-264. Harrison RAP, Vickers SE, 1990: Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J. Reprod. Fertil. 88 343–352. Heckenbichler S, Deichsel K, Peters P, Aurich C, 2010: Quality and fertility of cooledshipped stallion semen at the time of insemination. Theriogenology in press. Hillman G, 1971: Fortlaufende photometrische Messung der sauren Prostataphosphatase-Aktivität (Continuous photometric measurement of prostate acid phosphatase activity). Z Klin. Chem. Klin. Biochem. 9 273-274. 73 Hoffmann B, Landeck A, 1999: Testicular endocrine function, seasonality and semen quality of the stallion. Anim. Reprod. Sci. 57 89-98. Hoshiba H, Sinowatz F, 1998: Immunohistochemical localization of the spermadhesin AWN-1 in the equine male genital tract. Anat. Histol. Embryol. 27 351-353. Hough SR, Parks JE, 1994: Platelet-activating factor acetylhydrolase activity in seminal plasma from the bull, stallion, rabbit and rooster. Biol. Reprod. 50 912-916. Huleihel M, Lunenfeld E, Horowitz S, Levy A, Potashnik G, Mazor M, Glezerman M, 1999: Expression of IL-12, IL-10, PGE2, sIL-2R and sIL-6R in seminal plasma of fertile and infertile men. Andrologia 31 283-8. Ionata LM, Anderson TM, Pickett BW, Heird JC, Squires EL, 1991: Effect of supplementary sexual preparation on semen characteristics of stallions. Theriogenology 36 923-937. James RW, Heywood R, Street AE, 1979: Biochemical observations on beagle dog semen. Vet. Rec. 104 480-482. Janett F, Thun R, Niederer K, Burger D, Hässig M, 2003: Seasonal changes in semen quality and freezability in the Warmblood stallion. Theriogenology 60 453-461. Janett F, Burkhardt C, Burger D, Imboden I, Hässig M, Thun R, 2005: Influence of repeated treadmill exercise on quality and freezability of stallion semen. Theriogenology 65 1737-1749. Jasko DJ, Moran DM, Farlin ME, Squires EL, 1991: Effect of seminal plasma dilution or removal on spermatozoa motion characteristics of cooled semen. Theriogenology 35 10591067. Jasko DJ, Hathaway VL, Schaltenbrand VL, Simper WD, Squires EL, 1992: Effect of seminal plasma and egg yolk on motion characteristics of cooled stallion spermatozoa. Theriogenology 37 1241-1252. Jauhiainen A, Vanha-Perttula T, 1986: -glucuronidase in the bull seminal plasma and reproductive organs. Enzyme 35 137-146. Jobim MIM, Bustamante Filho IC, Trein C, Wald VB, Gregory RM, Mattos RC, 2005: Equine seminal plasma proteins related with fertility. Anim. Reprod. Sci. 89 305-308. Jonsson HT, Middleditch BS, Desiderio DM, 1975: Prostaglandins in human seminal fluid: two novel compounds. Science 187 1093-1094. 74 Kaczmarek MM, Krawczynski K, Blitek A, Kiewisz J, Schams D, Ziecik AJ, 2010: Seminal plasma affects prostaglandin synthesis in the porcine oviduct. Theriogenology. 2010 in press. Kareskoski AM, Reilas T, Calvete JJ, Sanz L, Rodriguez-Martinez H, Katila T, 2005a: Proteins in fractionated stallion seminal plasma. Reprod. Domest. Anim. 40 346. Kareskoski AM, Reilas T, Sankari S, Andersson M, Katila T, 2005b: Composition of fractionated stallion ejaculates. Anim. Reprod. Sci. 89 228. Kareskoski M, Katila T, 2008: Components of stallion seminal plasma and the effects of seminal plasma on sperm longevity. Anim. Reprod. Sci. 107 249-256. Kareskoski M, Kindahl H, Andersson M, Katila T, 2008a: Prostaglandin E2 concentrations and sperm motility in fractionated stallion ejaculates. Anim. Reprod. Sci. 107 326. Kareskoski M, Johannisson A, Rodriguez-Martinez H, Katila T, 2008b: Sperm chromatin integrity in fractionated stallion ejaculates. Reprod. Dom. Anim. 43 105. Kareskoski M, Rivera del Alamo MM, Reilas T, Katila T, 2009: SDS-PAGE of seminal proteins in fractionated stallion ejaculates. Reprod. Domest. Anim. 44 110. Katila T, 1997: Procedures for handling fresh stallion semen. Theriogenology 48 1217-27. Katila T, Karlsson M, Reilas T, Andersson M, Kaikkonen R, Koskinen E, 2001: Motility and viability of fractionated stallion ejaculates after 24 h of cooled storage. Havemeyer Foundation Monograph Series 5 3-5. Katila T, Andersson M, Reilas T, Koskinen E, 2002: Post-thaw motility and viability of fractionated and frozen stallion ejaculates. Theriogenology 58 241-244. Katila T, Reilas T, Güvenc K, Alm K, Andersson M, 2003: The effect of seminal plasma on motility characteristics and viability of spermatozoa after cooled storage. Havemeyer Foundation Monograph Series 13 3-5. Katila T, Kareskoski M, Akcay E, Reilas T, Koskinen E, Calvete JJ, 2006: Seminal plasma studies in stallions. Havemeyer Foundation Monograph Series 18 3-5. Katila T, Kareskoski M, 2006: Components of stallion seminal plasma and their influence on spermatozoa. Pferdeheilkunde 22 193-200. Katila T, Kareskoski AM, Venhoranta H, Virtala A-M, 2010a: The proportion of seminal plasma and the outcome of inseminations with transported stallion semen. Anim. Reprod. Sci. 121 194-195. 75 Katila T, Reilas T, Nivola K, Peltonen T, Virtala AM, 2010b: A 15-year survey of reproductive efficiency of Standardbred and Finnhorse trotters in Finland - descriptive results. Acta Vet. Scand. 52 40. Karow AM, Gilbert WB, Black JB, 1992: Effects of temperature, potassium concentration, and sugar on human spermatozoa motility: A cell preservation model from reproductive medicine. Cryobiology 29 250-254. Kenney RM, Bergman RV, Cooper WL, Morse GW, 1975: Minimal contamination techniques for breeding mares: techniques and preliminary findings. In: Proc. 21st Ann. Conv. Am. Assoc. Equine Pract. 327-336. Kenney RM, Hurtgen J, Pierson R, Witherspoon D, Simons J, 1983: Society for Theriogenology Manual for Clinical Fertility Evaluation of the Stallion. Society for Theriogenology, Hastings, NE 100. Killian JG, Chapman DA, Rogowski LA, 1993: Fertility-associated proteins in Holstein bull seminal plasma. Biol. Reprod. 49 1202-1207. Kosiniak K, 1975: Characteristics of the successive jets of ejaculated semen of stallions. J. Reprod. Fertil. Suppl. 23 59-61. Koskinen E, Karlsson M, Reilas T, Sankari S, Esala A-L, Katila T, 2002: Catalase activity and total protein in fractionated stallion seminal plasma. Theriogenology 58 337-340. Kotilainen T, Huhtinen M, Katila T, 1994: Sperm-induced leukocytosis in the equine uterus. Theriogenology 41 629-636. Kozdrowski R, Dubiel A, 2004: The effect of season on the properties of wild boar (Sus scrofa L.) semen. Anim. Reprod. Sci. 80 281-289. Kramer JW, Hoffmann WE, 1997: Clinical enzymology. In: Clinical biochemistry of domestic animals. 5th ed. Ed: Kaneko JJ. Academic Press, San Diego, USA. pp. 315-317. Kücük T, Sözen E, Buluç B, 2008: Effect of heat-induced hypermotility on pregnancy rate in intrauterine insemination for male factor infertility associated with asthenospermia: a prospective, randomized, controlled study. J. Assist. Reprod. Genet. 25 235-238. Kuisma P, Andersson M, Koskinen E, Katila T, 2006: Fertility of frozen-thawed stallion semen cannot be predicted by the currently used laboratory methods. Acta Vet. Scand. 48 14. Kutzler MA, Solter PF, Hoffmann WE, Volkmann DH, 2003: Characterization and localization of alkaline phosphatase in canine seminal plasma and gonadal tissues. Theriogenology 60 299-306. 76 Lapointe S, Ahmad I, Buhr MM, Sirard M-A, 1996: Modulation of postthaw motility, survival, calcium uptake, and fertility of bovine sperm by magnesium and manganese. J. Dairy Sci. 79 2163-2169. Leyton L, LeGuen P, Bunch D, Saling PM, 1992: Regulation of mouse gamete interaction by a sperm tyrosine kinase. Cell. Biol. 89 11692-11695. Lindeberg H, Karjalainen H, Koskinen E, Katila T, 1999: Quality of stallion semen obtained by a new semen collection phantom (Equidame) versus a Missouri artificial vagina. Theriogenology 51 1157-1173. Linko-Löppönen S, Mäkinen M, 1985: A microtiter plate assay for N-acetyl- -Dglucosaminidase using a fluorogenic substrate. Anal. Biochem. 148 50-53. Lopez ML, Grez P, Gribbel I, Bustos-Obregon E, 1989: Cytochemical and ultrastructural characteristics of the stallion epididymis (Equus caballus). J. Submicrosc. Cytol. Pathol. 21 103-120. Love C, 1992: Semen collection techniques. In: Stallion management, Eds. T.L.Blanchard and D.D. Varner. Vet. Clin. N.A.: Equine Pract. 8 111-128. Love CC, Kenney RM, 1998: The relationship of increased susceptibility of sperm DNA to denaturation and fertility in the stallion. Theriogenology 50 955-972. Love CC, Brinsko SP, Rigby SL, Thompson JA, Blanchard TL, Varner DD, 2005: Relationship of seminal plasma level and extender type to sperm motility and DNA integrity. Theriogenology 63 1584-1591. Magdaleno L, Gasset M, Varea J, Schambony AM, Urbanke C, Raida M, Töpfer-Petersen E, Calvete JJ, 1997: Biochemical and conformational characterisation of HSP-3, a stallion seminal plasma protein of the Cysteine-Rich Secretory Protein (CRISP) family. FEBS Lett. 420 179-185. Magistrini M, Seguin F, Beau P, Akoka S, Le Pape A, Palmer E, 1995: 1H nuclear magnetic resonance analysis of stallion genital tract fluids and seminal plasma: contribution of the accessory sex glands to the ejaculate. Biol. Reprod. Mono. 1 599-607. Magistrini M, Guitton E, Levern Y, Nicolle JC, Vidament M, Kerboeuf D, Palmer E, 1997: New staining methods for sperm evaluation estimated by microscopy and flow cytometry. Theriogenology 48 1229-35. Magistrini M, Lindeberg H, Koskinen E, Beau P, Seguin F, 2000: Biophysical and 1H magnetic resonance spectroscopy characteristics of fractionated stallion ejaculates. J. Reprod. Fert. Suppl. 56 101-110. 77 Mahan DE, Doctor BP, 1979: A radioimmune assay for human prostatic acid phosphataselevels in prostatic disease. Clin. Biochem. 12 10-17. Mann T, Leone E, Polge C, 1956: The composition of the stallion’s semen. J. Endocrin. 13 279-290. Mann T, Minotakis CS, Polge C, 1963: Semen composition and metabolism in the stallion and jackass. J. Reprod. Fertil. 5 109-122. Marquez B, Suarez SS, 2004: Different signaling pathways in bovine sperm regulate capacitation and hyperactivation. Biol. Reprod. 70 1626-1633. Massanyi P, Trandzik J, Nad P, Toman R, Skalicka M, Korenekova B, 2003: Seminal concentrations of trace elements in various animals and their correlations. Asian J. Androl. 5 101-104. Meseguer M, Garrido N, Martínez-Conejero JA, Simón C, Pellicer A, Remohí J, 2004: Relationship between standard semen parameters, calcium, cholesterol contents, and mitochondrial activity in ejaculated spermatozoa from fertile and infertile males. J. Assist. Reprod. Genet. 21 445-451. Métayer S, Dacheux F, Dacheux J-L, Gatti J-L, 2002: Comparison, characterization, and identification of proteases and protease inhibitors in epididymal fluids of domestic mammals. Matrix metalloproteinases are major fluid gelatinases. Biol. Reprod. 66 12191229. Moore AI, Squires EL, Graham JK, 2005: Effect of seminal plasma on the cryopreservation of equine spermatozoa. Theriogenology 63 2372-2381. Morrell JM, Johannisson A, Dalin A-M, Hammar L, Sandebert T, Rodriguez-Martinez, 2008: Sperm morphology and chromatin integrity in Swedish warmblood stallions and their relationship to pregnancy rates. Acta Vet. Scand. 50 2. Mungan NA, Mungan G, Basar MM, Baykam M, Atan A, 2001: Effect of seminal plasma calcitonin levels on sperm mobility. Arch. Androl. 47 113-117. Nickel R, Schummer A, Seiferle E, 1979: The viscera of the domestic mammals. 2nd revised ed. Verlag Paul Parey, Berlin , Germany. pp. 317-345. Owen DH, Katz DF, 2005: A review of the physical and chemical properties of human semen and the formulation of a semen stimulant. J. Androl. 26 459-469. Padilla AW, Foote RH, 1991: Extender and centrifugation effects on the motility patterns of slow-cooled stallion spermatozoa. J. Anim. Sci. 69 3308-3313. 78 Parks JE, Lynch DV, 1992: Lipid composition and thermotropic phase behavior of boar, bull, stallion, and rooster sperm membranes. Cryobiology 29 255-266. Parlevliet JM, Tremoleda JM, Cheng FP, Pycock JF, Colenbrander B, 1997: Influence of semen, extender and seminal plasma on the defense mechanism of the mare’s uterus. Pferdeheilkunde 13 540. Pesch SK, 2005: Licht- und elektronenmikroskopische Untersuchungen am Hengstejakulat sowie biochemische Analysen des Seminalplasmas (Light- and electrone microscopic studies on stallion ejaculate and biochemical analyses of seminal plasma). Dissertation, Justus-Liebig-Universität Giessen, Germany. Pesch S, Bergmann M, Bostedt H, 2006: Determination of some enzymes and macro- and microelements in stallion seminal plasma and their correlations to semen quality. Theriogenology 66 307-313. Pickett BW, Faulkner LC, Seidel GE, Berndtson WE, Voss JL, 1976: Reproductive physiology of the Stallion. IV. Seminal and behavioral characteristics. J. Anim. Sci. 43 617-625. Polakoski KL, Kopta M, 1982: Seminal plasma. In: Biochemistry of mammalian reproduction. Eds: Zaneveld LJD, Chatterton RT. John Wiley & Sons, NY, USA. pp. 89117. Portus BJ, Reilas T, Katila T, 2005: Effect of seminal plasma on uterine inflammation, contractility and pregnancy rates in mares. Eq. Vet. J. 37 515-519. Pruitt JA, Arns MJ, Pool KC, 1993: Seminal plasma influences recovery of equine spermatozoa following in vitro culture (37 ºC) and cold-storage (5 ºC). Theriogenology 39 291. Rathi R, Colenbrander B, Bevers MM, Gadella BM, 2001: Evaluation of in vitro capacitation of stallion spermatozoa. Biol Reprod. 65 462-70. Reinert M, Calvete JJ, Sanz L, Mann K, Töpfer-Petersen E, 1996: Primary structure of stallion seminal plasma protein HSP-7, a zona-pellucida binding protein of the spermadhesin family. Eur. J. Biochem. 242 636-640. Rethinaswamy A, Yang C-H, Srivastava PN, 1994: Purification and characterization of glucuronidase from bull seminal plasma and its role in fertilization. Mol. Reprod. Dev. 38 404-409. Rigby SL, Brinsko SP, Cochran M, Blanchard TL, Love CC, Varner DD, 2001: Advances in cooled semen technologies. Seminal plasma and semen extender. Anim. Reprod. Sci. 68 171-180. 79 Rossato M, Balercia G, Lucarelli G, Foresta C, Mantero F, 2002: Role of seminal osmolarity in the regulation of human sperm motility. Int. J. Androl. 25 230-235. Rota A, Furzi C, Panzani D, Camillo F, 2004: Studies on motility and fertility of cooled stallion spermatozoa. Reprod. Domest. Anim. 39 103-9. Saalmann A, Munz S, Ellerbrock K, Ivell R, Kirchhoff C, 2001: Novel sperm-binding proteins of epididymal origin contain four fibronectin type II-modules. Mol. Reprod. Dev. 58 88-100. Scandinavian Society for Clinical Chemistry and Clinical Physiology, 1974: Recommended methods for the determination of four enzymes in blood. Scand. J. Clin. Lab. Invest. 33 291-306. Schambony A, Gentzel M, Wolfes H, Raida M, Neumann U, Töpfer-Petersen E, 1998: Equine CRISP-3: Primary structure and expression in the male genital tract. Biochim. Biophys. Acta. 1387 206-216. Shimokawa K, Katayama M, Matsuda Y, Takahashi H, Hara I, Sato H, Kaneko S, 2002: Matrix metalloproteinase (MMP)-2 and MMP-9 activities in human seminal plasma. Mol. Hum. Reprod. 8 32-36. Sieme H, Katila T, Klug E, 2004: Effect of semen collection practices on sperm characteristics before and after storage and on fertility of stallions. Theriogenology 61 769784. Sorensen MB, Bergdahl IA, Hjollund NHI, Bonde JPE, Stoltenberg M, Ernst E, 1999: Zinc, magnesium and calcium in human seminal fluid: relations to other semen parameters and fertility. Mol. Hum. Reprod. 5 331-337. Sosa MA, Barbieri MA, Bertini F, 1991: Binding of beta-galactosidase from rat epididymal fluid to the sperm surface by high-affinity sites different from phosphomannosyl receptors. J. Reprod. Fert. 93 279-285. Sostaric E, Aalberts M, Gadella BM, Stout TA, 2008: The roles of the epididymis and prostasomes in the attainment of fertilizing capacity by stallion sperm. Anim. Reprod. Sci. 107 237-248. Stornelli A, Arauz M, Baschard H, de la Sota R-L, 2003: Unilateral and bilateral vasectomy in the dog: alkaline phosphatase as an indicator of tubular patency. Reprod. Dom. Anim. 38 1-4. Suzuki K, Asano A, Eriksson B, Niwa K, Nagai T, Rodriguez-Martinez H, 2002: Capacitation status and in vitro fertility of boar spermatozoa: effects of seminal plasma, cumulus-oocyte-complexes-conditioned medium and hyaluronan. Int. J. Androl. 25 84-93. 80 Tanaka M, Kishi Y, Takanezawa Y, Kakehi Y, Aoki J, Arai H, 2004: Prostatic acid phosphatase degrades lysophosphatidic acid in seminal plasma. FEBS Lett. 571 197-204. Tappel A, 2005: Lysosomal and prostasomal hydrolytic enzymes and redox processes and initiation of prostate cancer. Med. Hypotheses 64 1170-1172. Tibary A, 2007: Stallion reproductive behavior. In: Current therapy in equine reproduction. Eds: Samper JC, Pycock JF, McKinnon A. Saunders Elsevier, St. Louis, USA. pp. 174-175. Tischner M, Kosiniak K, Bielanski W, 1974: Analysis of pattern of ejaculation in stallions. J. Reprod. Fert. 41 329-335. Todd P, Arns MJ, Chenoweth P, Schultz B, 2001: Influence of seminal plasma and processing on cold-stored stallion spermatozoa. Anim. Reprod. Sci. 68 335-336. Troedsson MHT, Franklin RK, Crabo BG, 1999: Suppression of PMN-chemotaxis by different molecular weight fractions of equine seminal plasma. Pferdeheilkunde 15 568-573. Troedsson MHT, Lee C-S, Franklin RD, Crabo BG, 2000: The role of seminal plasma in post-breeding uterine inflammation. J. Reprod. Steril. Suppl. 56 341-349. Troedsson MHT, Alghamdi AS, Mattisen J, 2002: Equine seminal plasma protects the fertility of spermatozoa in an inflamed uterine environment. Theriogenology 58 453-456. Troedsson MHT, Desvousges A, Alghamdi AS, Dahms B, Dow CA, Hayna J, Valesco R, Collahan PT, Macpherson ML, Pozor M, Buhi WC, 2005: Components in seminal plasma regulating sperm transport and elimination. Anim Reprod Sci. 89 171-86. Troedsson MHT, Doty A, Macpherson ML, Connor MC, Verstegen JP, Pozor MA, Buhi WC, 2010: CRISP-3 in equine seminal plasma is involved in selective uterine sperm transport. Anim. Reprod. Sci. 121 192-193. Turner RMO, McDonnell SM, 2003: Alkaline phosphatase in stallion semen: characterization and clinical applications. Theriogenology 60 1-10. Töpfer-Petersen E, Waberski D, Hess O, Bellair S, Schambony A, Ekhlasi-Hundrieser M, Gentzel M, Reineke A, 1998: Bedeutung des Seminalplasmas für die Befruchtung – ein kurzer Überblick (The role of seminal plasma in fertilization – a short overview). Tierärztl. Umschau 53 447-454. Töpfer-Petersen E, Ekhlasi-Hundrieser M, Kirchhoff C, Leeb T, Sieme H, 2005: The role of stallion seminal plasma proteins in fertilisation. Anim. Reprod. Sci. 89 159-170. Varner DD, Blanchard TL, Love CC, Garcia MC, Kenney RM, 1987: Effects of semen fractionation and dilution ratio on equine spermatozoa motility parameters. Theriogenology 28 709-723. 81 Varner DD, 2008: Developments in stallion semen evaluation. Theriogenology 70 448462. Voge JL, Bliss SB, Hayden SS, Teague SR, Blanchard TL, Brinsko SP, Love CC, Varner DD, 2010: Cooled storage of semen with high or low sperm concentrations: effects on sperm motility, membrane integrity, and chromatin quality. Anim. Reprod. Sci. 121 210211. Watson PF, 1975: Use of a Giemsa stain to detect changes in acrosomes of frozen ram spermatozoa. Vet. Rec. 97 12-15. Webb RL, Evans JW, Arns MJ, Webb GW, Taylor TS, Potter GD, 1990: Effects of vesiculectomy on stallion spermatozoa. Eq. Vet. Sci. 10 218-224. Webb GW, Arns MJ, 1995: Influence of modified Tyrode’s media on motility of coldstored stallion spermatozoa. J. Equine Vet. Sci. 10 441-444. Weber JA, Freeman DA, Vanderwall DK, Woods GL, 1991: Prostaglandin E2 hastens oviductal transport of equine embryos. Biol Reprod. 45 544-6. Weber JA, Woods GL, 1993: Ultrasonographic measurement of stallion accessory sex glands and excurrent ducts during seminal emission and ejaculation. Biol. Reprod. 49 267273. Wong WY, Flik G, Groenen PMW, Swinkels DW, Thomas CMG, Copius-Peereboom JHJ, Merkus HMWM, Steegers-Theunissen RPM, 2001: The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod. Toxicol. 15 131–136. Woods J, Rigby S, Brinsko S, Stephens R, Varner D, Blanchard T, 1999: Effect of intrauterine treatment with prostaglandin E2 prior to insemination of mares in the uterine horn or body. Theriogenology 53 1827-1836. Wrench N, Pinto CR, Klinefelter GR, Dix DJ, Flowers WL, Farin CE, 2010: Effect of season on fresh and cryopreserved stallion semen. Anim. Reprod. Sci.119 219-227. Yanagimachi R, Usui N, 1974: Calcium dependence of the acrosome reaction and activation of guinea pig spermatozoa. Exp. Cell. Res. 89 161-174. Zini A, de Lamirande E, Gagnon C, 1993: Reactive oxygen species in semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int. J. Androl. 16 183-188. 82 Original articles 83