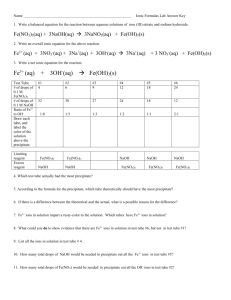
Ionic Formulas Lab
advertisement
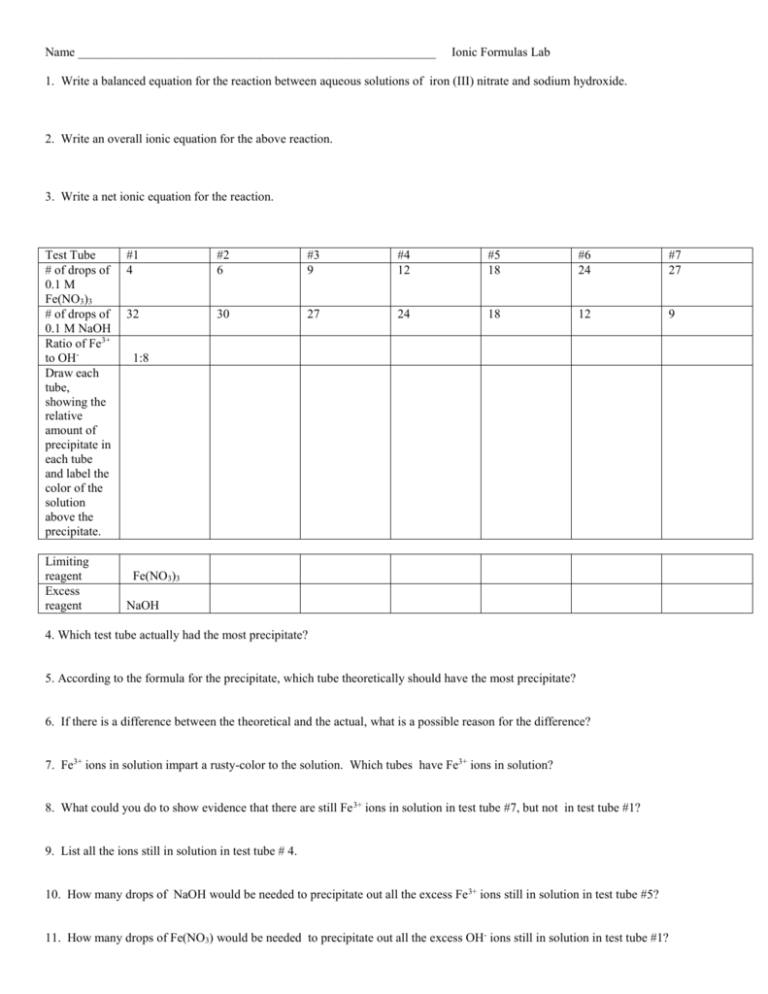
Name _________________________________________________________ Ionic Formulas Lab 1. Write a balanced equation for the reaction between aqueous solutions of iron (III) nitrate and sodium hydroxide. 2. Write an overall ionic equation for the above reaction. 3. Write a net ionic equation for the reaction. Test Tube # of drops of 0.1 M Fe(NO3)3 # of drops of 0.1 M NaOH Ratio of Fe3+ to OHDraw each tube, showing the relative amount of precipitate in each tube and label the color of the solution above the precipitate. Limiting reagent Excess reagent #1 4 #2 6 #3 9 #4 12 #5 18 #6 24 #7 27 32 30 27 24 18 12 9 1:8 Fe(NO3)3 NaOH 4. Which test tube actually had the most precipitate? 5. According to the formula for the precipitate, which tube theoretically should have the most precipitate? 6. If there is a difference between the theoretical and the actual, what is a possible reason for the difference? 7. Fe3+ ions in solution impart a rusty-color to the solution. Which tubes have Fe3+ ions in solution? 8. What could you do to show evidence that there are still Fe 3+ ions in solution in test tube #7, but not in test tube #1? 9. List all the ions still in solution in test tube # 4. 10. How many drops of NaOH would be needed to precipitate out all the excess Fe3+ ions still in solution in test tube #5? 11. How many drops of Fe(NO3) would be needed to precipitate out all the excess OH- ions still in solution in test tube #1?