UNIT 3 SCHEDULE FOR CHEMISTRY: Atomic Theory, Nuclear
advertisement

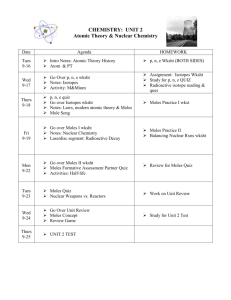
UNIT 3 SCHEDULE FOR CHEMISTRY: Atomic Theory, Nuclear Chemistry, & Moles Date Agenda HOMEWORK Student Learning Targets Thurs 10/1 Discuss Atomic Theory History NOTES: Modern View of the Atom p+, n, e- wksht Fri 10/2 Mon 10/5 Go over HW NOTES: Isotopes M&Mium Activity Quiz: The atom: p, n, e NOTES: Nuclear Chemistry & Radioactive Decay Isotopes wksht Review for p,n,e quiz Radioactive Decay Practice wksht I can define an atom. I can describe the modern view of the atom. I can explain how the atomic model has changed as new discoveries have occurred. I know the experiments used by scientists that led to the understanding of the atom. I can state the masses, charges, and locations of the three subatomic particles. I can use the periodic table to determine the number of protons, neutrons, and electrons, atomic mass, mass number, and symbol for any element. I can define the term isotope. I can determine the number of protons, neutrons, and electrons present in different isotopes of an element. I can calculate the average atomic mass for an element when given isotope data. I can write the symbols for alpha, beta, and gamma particles (including mass number and atomic number) I can balance nuclear equations. Tues 10/6 Go over nuclear equations HW NOTES: Nuclear Energy & Half-life Half-life Activity Wed 10/7 NOTES: Moles The Mole Song! Activity: Counting by Measuring Thurs 10/8 Review moles; go over HW Complete moles formative assessment with a partner Fri 10/9 Go over Moles II wksht Moles Quiz Go Over study guide Mon 10/13 UNIT TEST: Atomic Theory, Nuclear Chemistry, & Moles Radioactive Isotopes Reading Half-life wksht Moles I wksht Unit Study Guide (due Friday) Moles II wksht Finish Unit Study Guide Prepare for Moles quiz Prepare for Unit Test Collect notebooks to grade Bunsen burner lab, atomic history notes, and isotope lab I can define the term half-life. I can determine how much of a sample will remain after a given number of half-lives when provided the half-life and original sample size. I can explain the difference between fission and fusion. I can explain what a mole is used for in chemistry. I can determine the molar mass for an element or compound. I can perform mole to mass to particle conversions, using molar masses from the periodic table.