Chemistry
advertisement

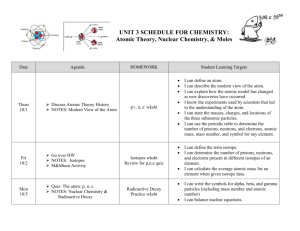
CHEMISTRY: UNIT 2 Atomic Theory & Nuclear Chemistry Date Agenda Tues 9-16 Intro Notes: Atomic Theory History Atom & PT Wed 9-17 Go Over p, n, e wksht Notes: Isotopes Activity: M&Mium Thurs 9-18 Fri 9-19 p, n, e quiz Go over Isotopes wksht Notes: Laws, modern atomic theory & Moles Mole Song Go over Moles I wksht Notes: Nuclear Chemistry Laserdisc segment: Radioactive Decay HOMEWORK p, n, e Wksht (BOTH SIDES) Assignment: Isotopes Wksht Study for p, n, e QUIZ Radioactive isotope reading & ques Moles Practice I wkst Moles Practice II Balancing Nuclear Rxns wksht Mon 9-22 Go over Moles II wksht Moles Formative Assessment Partner Quiz Activities: Half-life Tues 9-23 Moles Quiz Nuclear Weapons vs. Reactors Work on Unit Review Wed 9-24 Go Over Unit Review Moles Concept Review Game Study for Unit 2 Test Thurs 9-25 UNIT 2 TEST Review for Moles Quiz Chemistry Atomic Structure Student outcomes: Define an atom. Describe the five postulates of the Dalton’s Atomic Theory. Use a drawing to illustrate a model of the atom. Describe particles, locations, masses, year discovered, person who discovered them and experiment used. Define and distinguish between these numbers: Atomic number Mass number (and relation to isotopes) Atomic mass/Atomic weight Give the symbols for alpha, beta, and gamma particles (include mass number and atomic number) Balance nuclear equations involving alpha and beta decay Differentiate between fusion and fission Perform calculations with moles, particles, and grams Perform calculations to determine atomic mass (using isotope data) Describe the contributions of Democritus, Dalton, Rutherford, JJ Thomson, and Chadwick Terms/People to know: Democritus Atom Neutrons J.J. Thomson Nucleus Mole Mass number Binding Energy Beta decay Nuclear Reaction John Dalton Modern Atomic Theory electrons Gold Foil Experiment isotope atomic mass/weight fusion Chadwick Dalton’s Postulates protons Cathode Ray Tube Rutherford amu atomic number Avogardro’s number alpha decay fission half life