Problem Set 5 Solutions Chemistry 104a
advertisement
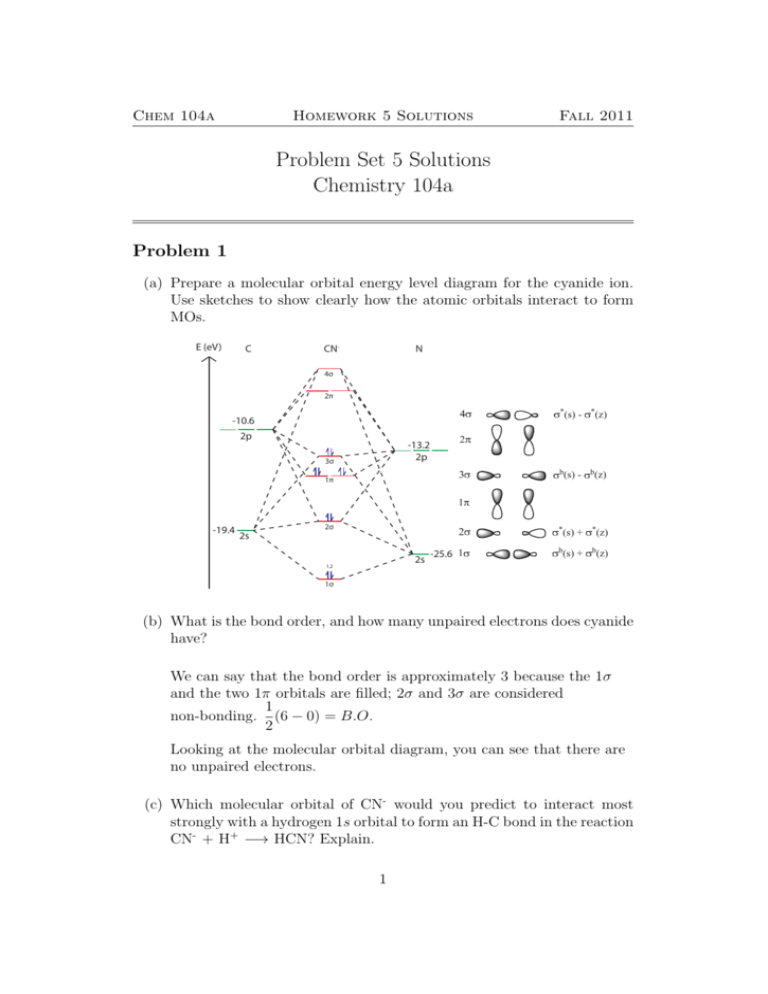
Chem 104a Homework 5 Solutions Fall 2011 Problem Set 5 Solutions Chemistry 104a Problem 1 (a) Prepare a molecular orbital energy level diagram for the cyanide ion. Use sketches to show clearly how the atomic orbitals interact to form MOs. E (eV) C CN- N 4σ 2π V -10.6 2p -13.2 2p 3σ ] EV 1π E] -19.4 2σ 2s 2s 1,2 -25.6 V E V E ] ] 1σ (b) What is the bond order, and how many unpaired electrons does cyanide have? We can say that the bond order is approximately 3 because the 1σ and the two 1π orbitals are filled; 2σ and 3σ are considered 1 non-bonding. (6 − 0) = B.O. 2 Looking at the molecular orbital diagram, you can see that there are no unpaired electrons. (c) Which molecular orbital of CN- would you predict to interact most strongly with a hydrogen 1s orbital to form an H-C bond in the reaction CN- + H+ −→ HCN? Explain. 1 Chem 104a Homework 5 Solutions Fall 2011 The 3σ (HOMO) of CN- would interact most with the LUMO of H+ 1s orbital, which has an energy of -13.6 eV, because they match in symmetry and in energy. Problem 2 Although KrF+ and XeF+ have been studied, KrBr+ has not yet been prepared. For KrBr+ : (a) Propose a molecular orbital diagram, showing the interactions of the valence shell s and p orbitals to form molecular orbitals. E (eV) NF B Kr 4σ 2π -12.5 -14.3 2p 2p 3σ 1π -24.1 2σ 2s 2s -27.5 1,2 1σ (b) Toward which atom would the HOMO be polarized? Why? The HOMO = 2π. The HOMO is more polarized toward the Br 4px and 4py . These Br 4px and 4py orbitals are closer in energy to the 2π MO than the Kr 4px and 4py . 2 Chem 104a Homework 5 Solutions Fall 2011 (c) Predict the bond order. Bond order is approximately 1 since the effects of the 2σ and 3σ cancel each other out just like the 1π and the 2π. (d) Which is more electronegative, Kr or Br? Explain your reasoning. Kr is more electronegative because its atomic orbitals are lower in energy than the corresponding Br atomic orbitals. As a result, you can see more filled orbitals have Kr character than Br character. Problem 3 Consider the molecule NF and the ions NF+ and NF- . Write the Lewis structure and the molecular orbital description of the ground state for each species. Determine which of the three species would be paramagnetic, and tell how many unpaired electrons there would be in each paramagnetic molecule. Predict the bond orders for all three species. E (eV) NF N F 4σ 11,13 2π 12 -13.2 2p 9,10 3σ 5,7 6,8 -18.6 2p 1π 4 3 -25.6 2s 2σ 1,2 2s 1σ 3 N F -40.2 -25.6 2s -13.2 5,7 6,8 3 1,2 4 9,10 2σ 1σ 2s 3σ 6,8 Homework 55,7 Solutions Chem 104a -18.6 2p 1π 2p -25.6 2π 2σ 3σ -40.2 2s -18.6 2p Fall 2011 1π NF + 1,2 4 3 -25.6 2s 2s -40.2 1σ 2σ N F 1,2 11 electrons -40.2 2s 1σ antibonding. The bond order is 8 electrons are bonding and 3 electrons are 5/2, and the molecule is paramagnetic due to one unpaired electron. NF N N N N N N F FF F FF 12 electrons 8 electrons are bonding and 4 electrons are antibonding. The bond order is 2, and the molecule is paramagnetic due to 2 unpaired electrons. NF- N F 13 electrons 8 electrons are bonding and 5 electrons are antibonding. The bond order is 3/2, and the molecule is paramagnetic due to one unpaired electron. 4 Chem 104a Homework 5 Solutions Fall 2011 Problem 4 The first ionization energies of BF, CO, and N2 are 11.06 eV, 14.01 eV, and 15.57 eV, respectively. Explain the increase in ionization energy for this isoelectronic series on the basis of atomic-orbital composition of the highest occupied molecular orbital. E (eV) B BF F C CO 4σ -8.3 2p O N2 N 4σ 2π 2π -10.6 2p 2π 3σ -14 N 4σ 3σ 2s 1π -18.6 2p 1π 3σ 1π -19.4 2s 2σ -15.8 2p -13.2 2p -13.2 2p 2σ 2σ -32.3 2s -25.6 2s -25.6 2s 1σ 1σ 1σ 2s -40.2 Note: Not showing s-p mixing for all 3 molecules. We can answer this question simply by inspecting the HOMO levels of each molecule because the HOMO level is a minimum value for ionization energy (IE). The HOMO level of BF is the highest since it is higher than -14.0 eV. The HOMO level of CO is slightly higher than the O 2p orbital (-15.8 eV) from s-p mixing; therefore, its HOMO level is lower than the HOMO level of BF, so its IE is larger. The HOMO level of N2 is the lowest because its 3σ bond is strongly bonding despite weak s-p mixing. Therefore, diatomic nitrogen has the largest IE. 5 4nb -18.6 3nb 1σ Chem 104a Homework 5 Solutions Fall 2011 Problem 5 2nb 1nb The species -40.2 of HF2 - is linear and symmetrical, F-H-F- , and has one of the strongest known systems of “hydrogen bonding”. (a) Draw atomic overlap representations for each of the σ and π orbitals of HF2 - . F H F σ π π (b) Draw a molecular-orbital energy-level scheme for HF2 - . Place the H atom 1s orbital on the left, the fluorine 2s and 2p atomic orbitals (or the appropriate SALCs) on the right, and the orbitals of HF2 - in the center. HF2- H F2- E (eV) 2σ -13.6 5nb 4nb -18.6 3nb 1σ 2nb -40.2 1nb 6 F σ H F Chem 104a Homework 5 Solutions Fall 2011 (c) Given the energy-level scheme derived in part (b), would you expect HF2 - to be diamagnetic or paramagnetic? diamagnetic (all electrons are paired up) (d) What is the H-F bond order in HF2 - ? 1 The total bond order = (2 − 0) = 1, which represents the bond order 2 over two H-F bonds. A single BOH-F would then be 1/2 Problem 6 Consider the borohydride anion, BH4 - , to be formed in the reaction H- + BH3 −→ BH4 D3h BH3 B H3 E (eV) 2E’ 2E’ 2A1’ 2A1’ -8.3 px py pz E’ E’ A2’’ 1A2” 1A2” -13.6 -14.0 E’ s A1 ’ A1’ 1E’ 1E’ 1A1’ 1A1’ Which orbital will the H- nucleophile attack on the BH3 molecule? Following the symmetry rules for chemical reactions laid out in pg. 324 328 of DG, we can argue that H- will act as a nucleophile and attack the LUMO on BH3 . The LUMO is a non bonding a2 ” orbital, which corresponds to the 2pz orbital of boron. 7 Chem 104a Homework 5 Solutions Fall 2011 Problem 7 Diborane, B2 H6 , has the structure shown. Using molecular orbitals (and showing appropriate orbitals on B and H from which the MOs are formed), explain how hydrogen can form “bridges” between two B atoms. (This type of bonding is discussed in Chapter 8 of MT) H H H H H B H H B H B H H B H H You can look at this problem as 2Hmolecules of BH3 , labeled (1) and (2), H H reacting with each other to make B2 H6B. B H H H H B H (1) H B HH (1) H B B H H (2) H H H HH H (2) B H H H B LUMO of each BH molecule Problem #6 shows us that the HOMO and 3 H will look like: (1) (2) H B H H H H HOMO B LUMO H 2 interactions LUMO (1) (1) H The goal now is to orient the HOMO Hon (1) with the LUMO on (2) to 2 interactions create one interaction. To create another interaction, we can orient the H H (1) (1) HOMO on (2) with the H LUMO on (1) to create another interaction while H the molecules areHOMO still in the same relative position. This can create aH H LUMO (2) (2) H “bridged” molecule. H HOMO H H (1) 2 interactions (2) (2) (1) H H H H (2) (2) 8 H H Chem 104a Homework 5 Solutions Fall 2011 Problem 8 In the bent geometry, we should consider s-p mixing. The Walsh diagram is made with s-p mixing. Please note that the position of 2a1 is system dependent. (a) NH2- N (c) E (eV) H-H E (eV) 2b2 2b2 3a1 -13.2 -13.6 (px) b2 (py) b1 (pz) a1 3a1 1b1 b2 a1 1b1 -13.2 -13.6 2a1 2a1 nonbonding (py) 2a1 (slightly bonding) 1b2 1b2 1b2 -25.6 PES -25.6 a1 (s) 1a1 1a1 1a1 (b) E (eV) 2σu 2b2 3a1 2σg 1b1 px py 2a1 1b2 1σu 1σg 1a1 C2v D∞h C2v is more stable because it provides the greater energy stabilization for 8 valence electrons Problem 9 Consider the hypothetical square-planar molecule XeH4 in the following coordinate system: 9 Chem 104a Homework 5 Solutions Fall 2011 Z Hc Xe Hd Y Hb Ha X (a) Write appropriate SALCs of the hydrogen 1s atomic orbitals which will overlap with each of the following xenon orbitals that can form molecular orbitals: 5s, 5px , 5py , and 5dx2 −y2 . D4h Γred E 4 2C4 0 C2 0 2C20 2 2C200 0 i 2S4 0 0 σh 4 Applying the reduction formula: 2σv 2 2σd 0 Z Γred = A1g + B1g + Eu Hc We can now apply the Projection operator to Hgenerate LGOs. Xe Hd b Don’t pay too much attention to the shading. The sign is arbitrary. Just know when the sign flips on a symmetry. Ha Y X P̂ A1g (Ha ) = 4(Ha + Hb + Hc + Hd ) Eu A1g B1g P̂ B1g (Ha ) = 4(Ha − Hb + Hc − Hd ) 10 5py with Eu A1g 5px with Eu 2 c Xe Hd Y Hb Ha X Homework 5 Solutions Z Chem 104a Fall 2011 Hc Y Hb Eu Z X B1g A1g Eu Hc P̂ Eu (Ha ) = 4(Ha − Hc ) Xe Hd Y Hb 5py with Eu Z Ha Eu X A1g B1g Xe Hd Eu Hb Ha X Eu B1g 5dx^2-y^2 with B1gEu Eu 5px with E u (b) Draw atomic-orbital overlaps with the appropriate Xe orbital for each of these SALCs. B1g A1g Eu 5py with Eu with A1g A1g Y P̂ Eu (Hb ) = 4(Hb − Hd ) 5dz^2 with A1g 5py with Eu A1g 5dx^2-y^2 with B1g 5px with Eu Hc A1g E 5px with u 5px with Eu 5py with Eu 5dx^2-y^2 with B1g 5dx^2-y^2 with B1g 5dz^2 with A1g 5dz^2 with (c) A1g Sketch a molecular-orbital energy-level diagram for XeH , showing the 4 Xe atomic orbitals on the left, the H 1s orbitals on the right, and the XeH4 orbitals in the center. 11 Chem 104a Homework 5 Solutions D4h Fall 2011 XeH4 Xe H4 E (eV) 2B1g 3A1g 5d xy yz xz B2g Eg Eg x2-y2 z2 A1g B1g 1B2g 1Eg 2Eu 5p px py pz Eu Eu A2u 1A2u B1g 2A1g -13.6 Eu A1g 1B1g 1Eu 5s A1g 1A1g (d) Indicate which molecular orbitals are occupied, and calculate the net Xe-H bond order, based on the molecular-orbital model. ab1g , ebu , bb1g and a∗1g are occupied. Bond Order = 1 1 · (8 − 2) = 3/4 4 2 Problem 10 Consider the molecule SiCl4 : (a) Draw the MO diagram for the molecule in a planar configuration. Use isolobal analogy so that we can use only s-orbitals for our LGO’s. This will make life easier. These orbitals will be take on the energy of the cholorine 3p (13.7 eV) orbitals. D4h Γred E 4 2C4 0 C2 0 2C20 2 12 2C200 0 i 2S4 0 0 σh 4 2σv 2 2σd 0 Cl Cl Chem 104a Si Homework 5 Solutions Cl Fall 2011 Cl Γred = A1g + B1g + Eu Cl Don’t pay too much attention to the shading. The sign is arbitrary. Just know when the sign flips on a symmetry. Cl Si Cl P̂ A1g (σ1 ) = 4(σ1 + σ2 + σ3 + σ4 ) Cl Cl Cl Si Eu Cl B1g A1g Cl P̂ B1g (H1 ) = 4(σ1 − σ2 + σ3 − σ4 ) Cl Cl Si 5py with Eu Cl E 5pux with Eu A1g Cl B1g A1g Eu P̂ Eu (H1 ) = 4(σ1 − σ3 ) Eu A1g A1g B1g P̂ Eu (H2 ) = 4(σ2 − σ4 ) 5px with Eu Eu A1g B1g 5py with Eu Eu 5px with Eu 13 5py with Eu 5px with Eu 5py with Eu Eu Chem 104a Homework 5 Solutions D4h Fall 2011 SiCl4 Si Cl4 E (eV) D4h SiCl4 Si E (eV) -7.7 -7.7 px py Eu Eu px py Eu Eu pz 1A2u A2u pz A2u Cl4 2Eu 2Eu 1A2u 2A1g B1g -13.7 s A1g -14.9 -13.7 -14.9 Eu 1B1g A1g 2A1g B1g 1Eu Eu 1B1g A1g s A1g 1Eu (b) It will be tetrahedral. E (eV) 1A1g 1A1g E (eV) 2Eu 2Td 2Eu 2Td 1A2u 1A2u 2A1 2A1g2A1g 2A1 1B1g 1B1g 1Eu 1Td 1Td 1Eu 1A1g 1A1 1A1g D4h Td D4h 1A1 Td 14