Notes 4
advertisement

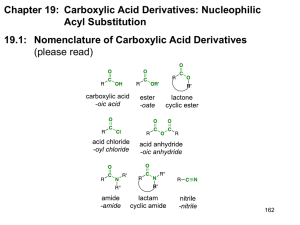
L TOPIC 4. CARBOXYLIC ACIDS AND THEIR DERIVATES: NUCLEOPHILIC ADDITION-ELIMINATION AT THE ACYL CARBON (Chapter 18) OBJECTIVES 1. Name carboxylic acids and acid derivatives: acyl chlorides, anhydrides, esters, amides and nitriles 2. Describe the preparation of acids and acid derivatives 3. Provide mechanisms for nucleophilic substitutions at acyl carbons bearing a leaving group and discuss implications of leaving group ability 4. Develop multistep syntheses CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 INTRODUCTION S:18.1 Review of carbonyl chemistry O C O C OH C C Nu Nu Nu H-Nu Review of nucleophilic substitution at sp3 carbons C Nu C L Nu Preview of Chapter 18 O C O C O C L Nu L Nu Nu FUNCTIONAL GROUPS general name O R C S:18.2 Prob:18.19,20 example with R=CH3 O alkanoic acid OH OH acetic acid H Ph [RCOOH] O R C Cl alkan acetyl chloride alkan acetic anhydride [RCOCl] O R C O O C R [(RCO)2] CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 alkane R R O C O C O OR' NR'2 alkan O alkan alkyl acetate acetamide O N R C N alkan acetonitrile Angelic acid defense substance for certain beetles, from the Swedish plant Garden Angelica (Archangelica officinalis) in1840s Traumatic Acid A plant hormone which causes injured cells to divide and help repair the trauma - Moronic acid CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 Properties of Carboxylic Acids Resonance stabilized conjugate base ⇒ carboxylic acids are more acidic than alcohols Hydrogen-bonding ⇒ carboxylic acids have higher boiling points than other molecules with similar molecular weights PREPARATION OF CARBOXYLIC ACIDS S:18.3 Prob:18.21-24 Review: Oxidation H R 1. KMnO4, -OH, Δ OH 2. H3O+ C R' R O or H 1. O3 2. H2O2 RCH2OH or R O C 1. KMnO4, -OH, Δ 2. H3O+ R R' C OH OH H H R C R CHEM2312: Spring 2008 O C O 1. KMnO4, -OH, Δ 2. H3O+ O C OH Notes: D.M. Collard, 2008 Hydrolysis of Nitriles NaOH, H2O, Δ or HCl, H2O, Δ R C N O R C OH Carboxylation of Grignard reagents R MgBr 1. CO2 2. H3O+ R O C OH O C R MgBr CHEM2312: Spring 2008 O Notes: D.M. Collard, 2008 Both of these methods increase the length of the chain by one carbon R MgBr R Br R O C OH R C N Problem [Solomons 18.21b] – How would you carry out the following transformation? CO2H Cl CHEM2312: Spring 2008 ? Cl Notes: D.M. Collard, 2008 ADDITION-ELIMINATION AT THE ACYL CARBON S:18.4 Overview O C Nu O C Nu O C L O C L Nu L L Nu O C Nu Relative Electrophilicity of Acid Derivatives R R R R CHEM2312: Spring 2008 O C O C O C O C Cl O Cl O C R O OR' NR'2 O C R OR' R O C NR'2 NR'2 Notes: D.M. Collard, 2008 Transformations of acids and acid derivatives O R C Cl O R O R C OH R C O C O O C R OR' O R C N R C NR'2 ? Problems 18.19-24 ? CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 L ACYL CHLORIDES S:18.5 Prob:18.24 Formation of acyl chlorides from carboxylic acids (and reverse reaction: hydrolysis) SOCl2 or PCl3 or PCl5 O R C OH Nucleophilic displacement of chloride from acyl chlorides: General Mechanism O R C Nu Cl Nu O C R Cl O R C Nu Reactions of acyl chlorides O O R C R' C R'OH Cl O R C O Na Cl O R C R'2NH Cl Acid chlorides are reactive enough that these reactions do not need to be catalyzed by acid or base. CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 ANHYDRIDES S:18.6 Prob:18.25 Formation of acid anhydrides from carboxylic acids (and reverse reaction: hydrolysis) O R C Δ OH Formation of acid anhydrides from acyl chlorides: see above Reactions of acid anhydrides O R C O O O R C C R'OH R O O C R'2NH R Hydrolysis: see above Acid anhydrides are reactive enough that these reactions do not need to be catalyzed by acid or base. O R C O O C O R R O R C O C Cl O C R Cl CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 ESTERS S:18.7 Prob:18.29,42 Acid catalyzed formation of esters from carboxylic acids – Fischer Esterification (and reverse reaction: hydrolysis) R O C R'OH H2SO4 Δ OH Formation of esters from acid chlorides: see above Formation of esters from acid anhydrides: see above Reactions of esters Amidation O R C R''2NH OR' Hydrolysis (saponification) O R C HO OR' Esters are quite unreactive – hydrolysis requires base (or acid) catalysis. CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 Saponification O H2C O animal fat HC O (CH2)7 O CH2CH3 (CH2)14CH3 O H2C O (CH2)7 (CH2)7CH3 NaOH, H2O O Na O Na O Na O H2C OH HC OH CH2CH3 O H2C OH glycerol (CH2)7 (CH2)14CH3 O (CH2)7 soap (CH2)7CH3 Problem - How could you prepare ethyl 4-aminobenzoate (benzocaine) ? O O H2N benzocaine O O H2N CHEM2312: Spring 2008 N novocaine Notes: D.M. Collard, 2008 Problem [Solomons 18.42] – Explain the following observations. OH Δ HO2C Δ OH HO2C ? H2O + ? No reaction AMIDES S:18.8A-F Prob:18.30,39 Formation of amides from carboxylic acids (and reverse reaction: hydrolysis) R C OH O O R'2NH Δ O OH NHR' NR' lactam A more common synthetic method makes use of a two step synthesis, via acyl chloride O O SOCl2 R'2NH C C R OH R NR'2 Formation of amides from acid anhydrides: see above Formation of amides from esters: see above CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 Problem - Propose a synthesis of N,N-diethyl-m-toluamide (DEET), the active ingredient in a number of commercial insect repellants. O N(CH2CH3)2 CH3 ? N-(1,1-dimethylethyl)-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide Type II 5α-reductase is an enzyme that metabolises testosterone (an androgen, or male sex hormone) into dihydrotestosterone, which is responsible for onset of male pattern baldness. Propecia® (finasteride) is a inhibitor of the enzyme, and an effective treatment for baldness., HN HO O tBuNH2 CH3 O CH3 H O CHEM2312: Spring 2008 N H H H Notes: D.M. Collard, 2008 Reactions of amides Hydrolysis O C R 1. NaOH, H2O Δ NR'2 2. H3O+ Amides are quite unreactive – hydrolysis requires base (or acid) catalysis. Dehydration O C R P2O5 Δ NH2 CONDENSATION POLYMERS Polyester O C HO Nylon O HO C OH Sb2O5 OH O C Δ O C O O n O OH HO O NH2 H2N O O O Δ H N O NH3 H3N O N H O n Proteins O H2N OH R CHEM2312: Spring 2008 enzyme O H N R n Notes: D.M. Collard, 2008 Poly(lactic Acid) O HO OH O O O O O O O O n ? Problems 18.24,25,29,30,39? CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 REACTIONS OF ACID DERIVATIVES WITH REDUCING AGENTS AND ORGANOMETALLICS Acid Chlorides Reduction O C Cl R R O C 1. LiAlH4 2. H3O+ 1. LiAlH4 R CH2 OH 2. H3O+ R O C OH LiAlH(t-BuO)3 Cl -78 °C Reaction with Organocopper Reagents R R O C O C 1. R'MgBr (or R'Li) Cl 2. H3O+ 1. R'2CuLi Cl 2. H3O+ O (CH3)2CuLi Cl CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 Problem - 3-Hexanone revisited. Provide a synthesis of 3-hexanone from alcohols with three of fewer carbon atoms. O previously HC CH Br OH O Br H ? Li O Esters Reduction R R O C O C 1. LiAlH4 OCH3 2. H3O+ 1. LiAlH4 R CH2 OH 2. H3O+ R O C OH 1. DIBAL-H -78oC OCH3 2. H3O+ Reaction with Grignard reagents O Ph O CH3 + H3C MgI 1. Et2O 2. H3O+ OH Ph CH3 CH3 via: CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 Problem - Propose a synthesis of 2-methyl-2-hexanol from pentanoic acid OH ? Amides Reduction – Synthesis of amines R O C 1. LiAlH4 NH(R)2 2. H3O+ R CH2 NH(R)2 Compare to the case with acids and esters (cleavage of C-O) bond) R CHEM2312: Spring 2008 O C 1. LiAlH4 OH(R) 2. H3O + R CH2 OH Notes: D.M. Collard, 2008 L NITRILES S:18.8G Formation and Reactions O O R C R OH C NR'2 R'=H R C N Reduction 1. LiAlH4 R C N 2. H3O+ R CH2 NH2 1. DIBAL-H -78oC R C N 2. H3O+ CHEM2312: Spring 2008 Notes: D.M. Collard, 2008 Reaction with Grignard Reagents C N 1. CH3MgBr 2. H2O Methadone binds to the opioid receptor in the brain, lowering the patient’s desire for heroin. N O C Ph CHEM2312: Spring 2008 N Ph 1. EtBr, Mg N 2. H3O+ Ph Ph Notes: D.M. Collard, 2008 SYNTHETIC STATEGIES Problem - Bis(2-ethylhexyl)phthalate is commonly used as a plasticizer for PVC. How could it be prepared on an industrial scale from inexpensive starting materials? O O O O Problem - Propose a synthesis of 3-chlorobenzonitrile Cl C CHEM2312: Spring 2008 N Prob:18.28,33, 34,45 ? ? Notes: D.M. Collard, 2008 Problem - Propose a synthesis of novocaine (procaine) O H2N O N ? http://www.corbis.com/ Spectroscopy Problem! (do problems 18.35,36) Elemental analysis: C, 72.0%; H, 6.7% ? IR: 1740 cm-1 1H CHEM2312: Spring 2008 NMR: δ 2.2 (s, 3H), 5.2 (s, 2H), 7.4 (br. S, 5H) Notes: D.M. Collard, 2008 Problem – The following compound was prepared at Georgia Tech as part of a National Institutes of Health study of novel treatments of cocaine addiction. How could this compound be prepared? [Hint: amides are reduced to amines with lithium Ph aluminum hydride, i.e., RCONH2 gives RCH2NH2] H OH H NH2 TOPIC 4 (CHAPTER 18) ON EXAM 4 Types of Questions - Predict the product of reactions, provide a mechanistic rationale to account for the formation of a particular product, naming acids and derivatives - Do the problems in the book; they are great examples of the types of problems on the exam! Preparing for Exam 4 - Get up-to-date NOW! - Work as many problems as possible. Do the problems first, then consult the solutions manual. - Work in groups, discuss chemistry, teach and test each other. - Do the “Learning Group Problem” at the end of the chapter. CHEM2312: Spring 2008 Notes: D.M. Collard, 2008