LAB 1-1 Household Chemicals
advertisement

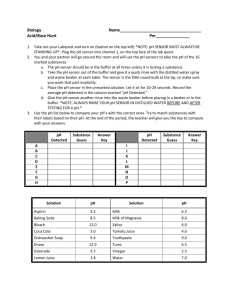
Name________________________________________________ Hour_________ General Chemistry Lab: Investigating Household Acids and Bases Objective: Categorize various substances as acids or bases and collect evidence to prove which are strong and which are weak. Substance Conductivity pH (with sensor) pH (with paper) Litmus paper (red or blue?) 1. dish soap 2. laundry soap 3. milk 4. coca cola 5. tomato juice 6. coffee 7. vinegar 8. orange juice 9. lemon juice 10. window cleaner 11. 0.1M HCl 12. 0.1M NaOH CAUTION: Even dilute acids and bases can cause burns! BE CAREFUL when handling these materials. If acid or base gets on your skin, immediately wash the area with soap and water. Procedures: Move from station to station to collect information about each acid or base. Leave the equipment at each station. Record your results in the data table. 1. Use the pH sensor as follows: Unscrew the cap on the storage solution and remove the pH sensor from the storage container. Slide the cap off of the pH sensor before using. Hold the pH sensor over the “waste” beaker and rinse it with distilled water. Dip the sensor in the solution to be tested and record the pH reading that appears on the computer screen. Hold the pH sensor over the “waste” beaker again and rinse it again with distilled water before returning to the storage solution. Screw the cap on the storage solution. (Remember: “RINSE, USE, RINSE!”) Return the pH probe to storage solution after use (DO NOT LET IT DRY OUT). 2. Use the conductivity sensor as follows: Make sure the switch on the black box is down (pointing to “0-2000” range) Use like the pH sensor. Use the “rinse, use, rinse” technique. 3. Use litmus and pH paper by dipping it directly into the solution. Discard of the used paper in the trash. Please do NOT put it in the sink or leave it on the counter. When you are finished, Please place the pH sensor inside the storage box like shown. This way, the sensor will be submerged in the storage solution. POST LAB QUESTIONS 1. Put each substance in one of the following categories: STRONG ACID STRONG BASE WEAK ACID WEAK BASE NEUTRAL 2. Are strong acids and bases good electrical conductors? Why/Why not? 3. What color is red litmus paper in acid? What color is red litmus in base? 4. What color is blue litmus paper in acid? What color is blue litmus paper in base? 5. What is the pH range for acids? 6. What is the pH range for bases?