BIOL 129 Mitosis and the Cell Cycle INTRODUCTION Cell division
advertisement

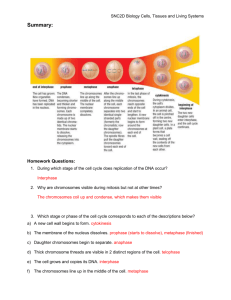
BIOL 129 Mitosis and the Cell Cycle INTRODUCTION Cell division is the process by which eukaryotic cells reproduce. The hallmark of the process is the apportionment of an identical genome to each daughter cell. Division of the nuclear contents is referred to as karyokinesis or, more commonly, mitosis. Nuclear division is usually followed by cytoplasmic division or cytokinesis, the apportionment of the cytosol and cytoplasmic organelles to the daughter cells as cell boundaries are formed. In this project, you will study mitosis in root tip cells of the broad bean, Vicia faba. It is a convenient and very popular system for studying mitosis because the chromosomes are very large, the diploid number is relatively low (2n = 12), and cell material is easily prepared. You will make squash preparations of the root tip cells and identify all the stages of mitosis. The Cell Cycle In the life cycle of a eukaryotic cell, mitosis is part of a larger program, the cell cycle. Actively dividing cells, such as in meristematic tissues of plants or in embryonic tissues, are alternately in mitosis and in interphase. It is during interphase that cell growth and DNA occur, the latter during the S period. There are two other periods in interphase, G1 (1st gap), which precedes DNA synthesis, and G2 (2nd gap), which follows it. A cell in G1 is considered to have the 2C level of DNA. During the S period, the amount of DNA doubles to the 4C level and remains there until the end of division. After cytokinesis, each daughter cell ends up with the 2C level of DNA. A mature eukaryotic cell that is destined not to undergo further division is usually arrested prior to DNA syntheses, G1. There is considerable variation both in the relative and actual duration of each stage depending on the organism, cell type, and environmental conditions. In meristematic cells of V. faba root tips, the cell cycle lasts about 19 hours. The specific durations of G1, S, G2, and mitosis have been determined from an autoradiographic analysis. Mitotic Stages In the interphase nucleus, the chromosomal material is dispersed as a rather homogeneous mass of fibers, referred to as chromatin. There may be several clumps of condensed, deeply stained heterochromatin, and there is always at least one nucleolus. In your preparations, nucleoli will appear as large, lightly stained, round or oval structures within the nucleus. The stages of mitosis are prophase, metaphase, anaphase, and telophase. While each mitotic stage is distinct, one stage does flow into the next so that the adjectives early and late are often appropriate. Prophase: Prophase begins when the individual chromosomes can first be discerned. Though the chromosomes are long and thin, the double nature of each is apparent. The two subunits, termed chromatids, are joined at a narrow chromosomal region known as the centromere or primary constriction. Throughout prophase, the chromosomes condense and shorten. The spindle assembles in late prophase, and the chromosomes migrate toward a median position between the poles, a process termed congression. These movements are effected by spindle fibers extending from the poles to the chromosomes. The spindle fibers, which are composed of microtubules, terminate within the kinetochore, a plate-like structure at the centromeric region of each chromatid. Other 2 spindle fibers extend from pole to pole. The other event that occurs at late prophase is the disassembly of the nucleoli and nuclear envelope. Metaphase: At metaphase, the fully condensed chromosomes are lined up on the metaphase plate, a plane that lies midway between the poles. Although all centromeres lie on the metaphase plate, the chromosome arms are still quite long and extend laterally to either side of the plate. Each metaphase chromosome has a characteristic morphology determined by its size and centromere location. Metacentric chromosomes have a median centromere and two arms of equal length. In acrocentrics, the centromere is very close to one end, while in telocentrics it is at the tip. In V. faba there are 5 pairs of acrocentric chromosomes and one pair of metacentric chromosomes; each metacentric is about twice as long as each acrocentric. Another morphological feature apparent in certain chromosomes is the secondary constriction. The secondary constriction is the very thin, lightly stained chromosomal region where a nucleolus forms at telophase. The chromosomal region distal to the secondary constriction is termed a satellite. In V. faba there is a secondary constriction in one pair of chromosomes. Anaphase: Anaphase is the climax of mitosis. The two chromatids part at the centromere and move to opposite poles, and, in addition, the poles move farther apart. The mechanism of anaphase movement is not fully understood but may involve spindle microtubules sliding past each other. The shape of the anaphase chromosome depends on the location of the centromere. The centromere leads the way to the pole so that a metacentric chromosome is V-shaped, a submetacentric J-shaped, and a telocentric rod-shaped. At late anaphase, the first sign of cytokinesis occurs with the formation of the phragmoplast. The phragmoplast is a collection of dense material in small Golgi vesicles associated with spindle fibers at the equatorial region. Telophase: Telophase commences when the two daughter sets of chromosomes reach the poles. Each daughter cell receives the same number of chromosomes as had been present in the parent cell, though each chromosome now has only one subunit. There is a general reversal of events that characterize prophase. The telophase chromosomes decondense and become "fuzzy," the spindle fibers disassemble, and the nucleoli and nuclear envelope reassemble, the latter from membranes of the endoplasmic reticulum. Cytokinesis proceeds with the accumulation and fusion of vesicles at the equatorial region. The fused membranes of the vesicles produce continuous plasma membranes for the adjacent daughter cells, and the contents of the vesicles form the cell plate, which eventually develops into the primary cell wall. Mitotic Index In any population of mitotically active cells, only some of the cells are in mitosis at any one time. The percentage of dividing cells is defined as the mitotic index. The approximate duration of mitosis can be obtained simply by multiplying the mitotic index by the total duration of the cell cycle. You will determine both the mitotic index and the duration of mitosis in V. faba root tips. For such an analysis, one should examine a cell population that is mitotically active. Since not all cells in the root apical meristem are 3 mitotically active, the values you obtain for the mitotic index and the duration of mitosis will be underestimates. Having an estimate for the duration of mitosis, you will then obtain estimates for the durations of the mitotic stages. Root Tip Preparation The beans were germinated and grown for one week under a lamp. They were uprooted and briefly rinsed in water. 5 mm terminal tips of secondary (lateral) roots were cut with a razor blade and then placed in freshly prepared Carnoy's fixative (absolute ethanol:glacial acetic acid, 3:1). The tips were fixed for 24 hrs, transferred to 70% ethanol and stored in a refrigerator at 4-5o C. Feulgen Squash The stages of mitosis are generally studied in squash preparations. Unlike sectioned material, where only a part of the cell may be present, each cell in a squash preparation contains all of the chromosomes. In addition, the squashing flattens the cells so that the chromosomes are more dispersed and are often in the same focal plane. You will start with fixed root tips that are ready for staining of the DNA by Schiff's reagent. Other cell structures, like the spindle, either do not stain with Schiff's reagent or have been destroyed by fixative or acid pretreatment. The HCL hydrolysis, which is part of the Feulgen reaction, also macerates or softens the plant fibers so cells squash flatter. There is no bisulfite rinse, as there is no need to verify the presence of DNA. After staining the intact root tip, transfer it to a slide for squashing. The squash is done in a drop of 45% acetic acid, which further softens the tissue. Cells are squashed by thumb pressure on the coverslip. PROCEDURES Feulgen Squash 1. Rinse the fixed root tips briefly in distilled water and then place in a beaker of 1 N HCl kept at 60oC for 10 min in a water bath. 2. Rinse the root tips briefly in distilled water and then place in a vial of Schiff's reagent, stoppered and kept in the dark, for 30 min. 3. Rinse the root tips in several changes of distilled water. The intensely stained distal tips contain the smaller, actively dividing cells. 4. Drain a root tip by touching it to a paper towel. Then, very gently rub the extreme tip of the root on the towel to remove the root cap. 4 5. Immediately place the tip on a labeled, alcohol-cleaned slide with a drop of acetic acid and, with a sharp, single-edge razor blade, cut off and discard all but the terminal 1 mm, which contains the dividing cells. Allow the tissue to remain the acetic acid for about 2 min. 6. Tap the root tissue with the flat end of a glass rod to produce a homogeneous suspension in the drop of acetic acid. Gently lower a coverslip on the preparation. 7. Before squashing, remove the excess liquid as follows. Place a piece of paper towel at one edge of the coverslip to draw out the excess liquid. Be very careful not move the coverslip laterally, as this will cause the cells to fold over on themselves, ruining the preparation. 8. Place a piece of paper towel over the coverslip and squash the preparation with the thumb. Use a rolling motion so that the liquid is pressed to the edges where the towel can absorb it. The pressure should be increased in successive rounds of squashing and not applied all at once. Again, do not move the coverslip laterally. 9. Seal edges of the coverslip with thin layer of Vaseline applied with a toothpick. Observations 1. Examine the preparation with the low power (10X) and high-dry (40X) objectives. Identify and draw a cell at interphase, prophase, metaphase, anaphase, and telophase. 2. Examine several interphase nuclei, noting the prominent nucleoli. How many nucleoli are there per nucleus? 3. Count 200 cells and determine the number of cells in the phases of mitosis and interphase. To do this, move the slide to a position with nicely-squashed cells. Count the number of cells in each mitotic phase and those in interphase. Then move the slide to a new position and repeat the procedure until the total number of cells counted is 200. This count represents a random sample of the entire population of cells, so do not search the slide for a specific mitotic phase. Just move the slide and count the cells in view. 4. Total the number of cells in mitosis and interphase for the class. Using the combined class data calculate the mitotic index. 5. From the value of the mitotic index and the known duration of the cell cycle in V. faba (19 hr), estimate the duration of mitosis. 6. Determine the relative frequency of each mitotic stage. A combined figure should be used for anaphase and telophase, since late anaphase and early telophase are difficult to distinguish. Using the combined class data, calculate the durations of these stages.