oxidation ladder.cdx - Master Organic Chemistry
advertisement

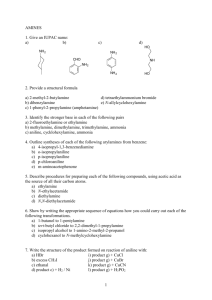
"Master Organic Chemistry" Summary Sheet - The Oxidation Ladder Note - this sheet is not meant to be comprehensive. Your course may provide additional material, or may not cover some of the reactions shown here. Your course instructor is the final authority. masterorganicchemistry.com June 2011. Version 1.0 Oxidation state of carbon +4 Notes: to keep things relatively simple, several common functional groups (amines, epoxides, ethers, and many more) have been omitted. CO2 Indicates oxidations Indicates reductions Neither oxidations nor reductions All alkyl halides are drawn as chlorides ("Cl"). For Br and I, the corresponding reagent containing those atoms should be employed. Carbon dioxide Oxidation here is defined as: loss of a C–H bond or gain of a C–O bond (or equivalent) Reduction here is defined as: gain of a C–H bond or loss of a C–O bond (or equivalent) H2O, acid ROH, acid O LiAlH4 +2 R HO Carboxylic acid O SOCl2 H2O or NaOH NH3 or other amine R Cl Acid halide O P2O5 R H2N N H2O, acid Amide O C R RO Ester Nitrile R H2O, acid R E D U C T I O N O X I D A T I O N amine, DCC NH3 or other amine mCPBA O3, H2O H2CrO4 or H2O2 H2CrO4 or KMnO4 H2O, H2SO4 or HgSO4, H2O, H2SO4 BH3, H2O2 O 0 NaNH2 Cl R NaNH2 R H Aldehyde NaBH4 or PCC LiAlH4 R Alkyne Cl Dihalide (Vicinal) HCl R Ketone Dihalide (Geminal) O3, Zn (or DMS) H2SO4, heat NaOH (SN2) -2 Alcohol (Primary) Cl PCl3 or SOCl2 R R base (e.g. NaOEt) Alkene Alkyl halide (Primary) Pd/C H2 NaOH (SN2) Cl HCl base (e.g. NaOEt) Mg, then acid PCC or H2CrO4 R Alkyl halide (Secondary) HCl OH R Alcohol (Secondary) OH R Alcohol (Tertiary) Mg, then acid Cl2, light –4 NaBH4 or LiAlH4 H2O, H2SO4 or Hg(OAc)2, H2O, then NaBH4 BH3, H2O2 R RMgCl or RLi O R H2, Lindlar's catalyst Cl2 O3, Zn (or DMS) Cl Cl H2SO4, heat HO RMgCl or RLi DIBAL Cl2, light R R Alkanes Omissions, Mistakes, Suggestions? james@writechem.com This sheet copyright 2011, James A. Ashenhurst masterorganicchemistry.com