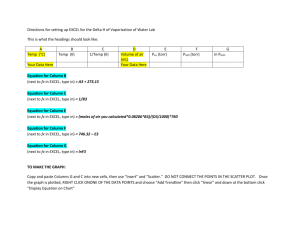
RSPT 2305: Section 9 CALCULATION OF GAS VOLUMES AT
advertisement
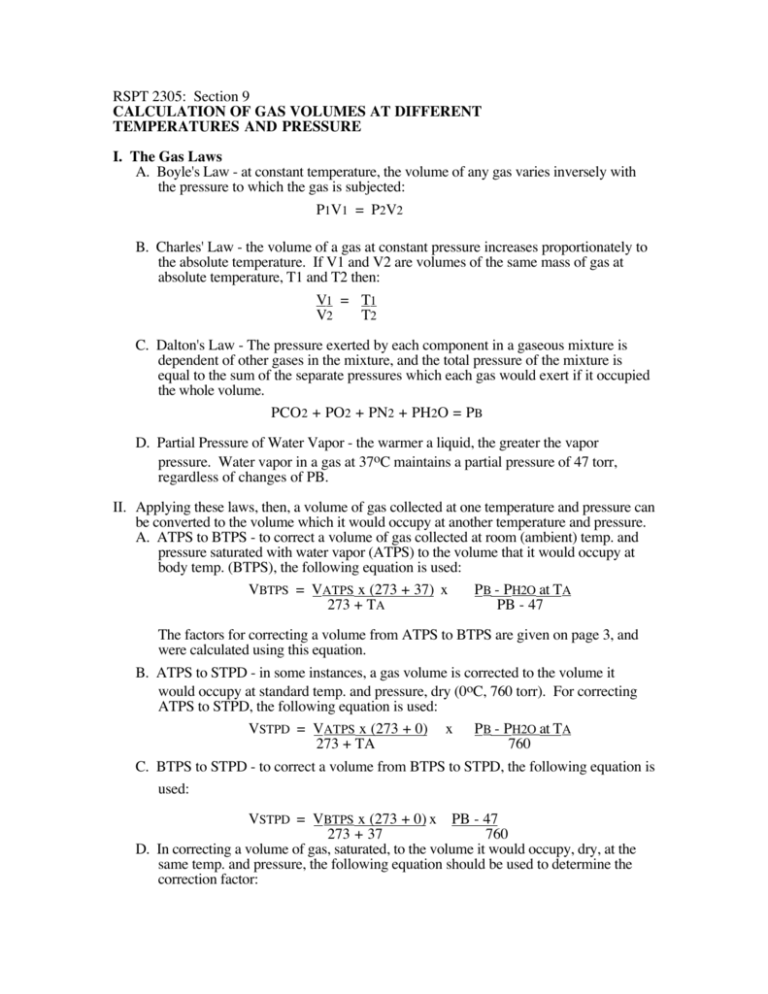
RSPT 2305: Section 9 CALCULATION OF GAS VOLUMES AT DIFFERENT TEMPERATURES AND PRESSURE I. The Gas Laws A. Boyle's Law - at constant temperature, the volume of any gas varies inversely with the pressure to which the gas is subjected: P1V1 = P2V2 B. Charles' Law - the volume of a gas at constant pressure increases proportionately to the absolute temperature. If V1 and V2 are volumes of the same mass of gas at absolute temperature, T1 and T2 then: V1 = T1 V2 T2 C. Dalton's Law - The pressure exerted by each component in a gaseous mixture is dependent of other gases in the mixture, and the total pressure of the mixture is equal to the sum of the separate pressures which each gas would exert if it occupied the whole volume. PCO2 + PO2 + PN2 + PH2O = PB D. Partial Pressure of Water Vapor - the warmer a liquid, the greater the vapor pressure. Water vapor in a gas at 37oC maintains a partial pressure of 47 torr, regardless of changes of PB. II. Applying these laws, then, a volume of gas collected at one temperature and pressure can be converted to the volume which it would occupy at another temperature and pressure. A. ATPS to BTPS - to correct a volume of gas collected at room (ambient) temp. and pressure saturated with water vapor (ATPS) to the volume that it would occupy at body temp. (BTPS), the following equation is used: VBTPS = VATPS x (273 + 37) x PB - PH2O at TA 273 + TA PB - 47 The factors for correcting a volume from ATPS to BTPS are given on page 3, and were calculated using this equation. B. ATPS to STPD - in some instances, a gas volume is corrected to the volume it would occupy at standard temp. and pressure, dry (0oC, 760 torr). For correcting ATPS to STPD, the following equation is used: VSTPD = VATPS x (273 + 0) x PB - PH2O at TA 273 + TA 760 C. BTPS to STPD - to correct a volume from BTPS to STPD, the following equation is used: VSTPD = VBTPS x (273 + 0) x PB - 47 273 + 37 760 D. In correcting a volume of gas, saturated, to the volume it would occupy, dry, at the same temp. and pressure, the following equation should be used to determine the correction factor: Section 9; Calculation of Gas Volumes 2 VDRY = VSAT x (PB - PH2O at ambient temp) PB Table 1 Water Vapor Pressure Gas Temp (oC) PH2O (torr) 20..................................................17.5 21..................................................18.7 22..................................................19.8 23..................................................21.1 24..................................................22.4 25..................................................23.8 26..................................................25.2 27..................................................26.7 28..................................................28.3 29..................................................30.0 30..................................................31.8 31..................................................33.7 32..................................................35.7 33..................................................37.7 34..................................................39.9 35..................................................42.2 36..................................................44.6 37..................................................47.0 Section 9; Calculation of Gas Volumes E. Factors to Convert Gas Volumes from Room Temperature Saturated (ATPS) to Volume Occupied at 37o C, Saturated (BTPS) Table 2 Conversion Factors ATPS TO BTPS - Amarillo, Texas Ambient Temperature (oC) Conversion Factor 20.....................................................1.108 21.....................................................1.101 22.....................................................1.097 23.....................................................1.091 24.....................................................1.086 25.....................................................1.080 26.....................................................1.073 27.....................................................1.067 28.....................................................1.062 29.....................................................1.055 30.....................................................1.049 31.....................................................1.042 32.....................................................1.034 33.....................................................1.028 34.....................................................1.020 35.....................................................1.014 36.....................................................1.007 37.....................................................1.000 Note: These factors have been calculated for a PB of 665 torr. Since the factors change very little from 675 to 655 torr, it is not necessary to correct for small changes in PB. However, if the PB varies greatly from 665 torr, the formula on page 1 (II, A) should be used to obtain the correct conversion factor. 3