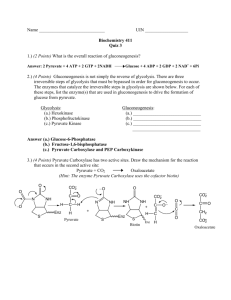
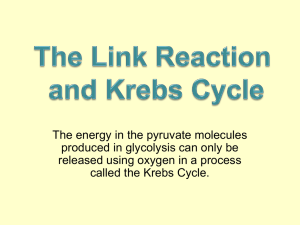
Regeneration of NAD+ Lactic Acid Fermentation
advertisement
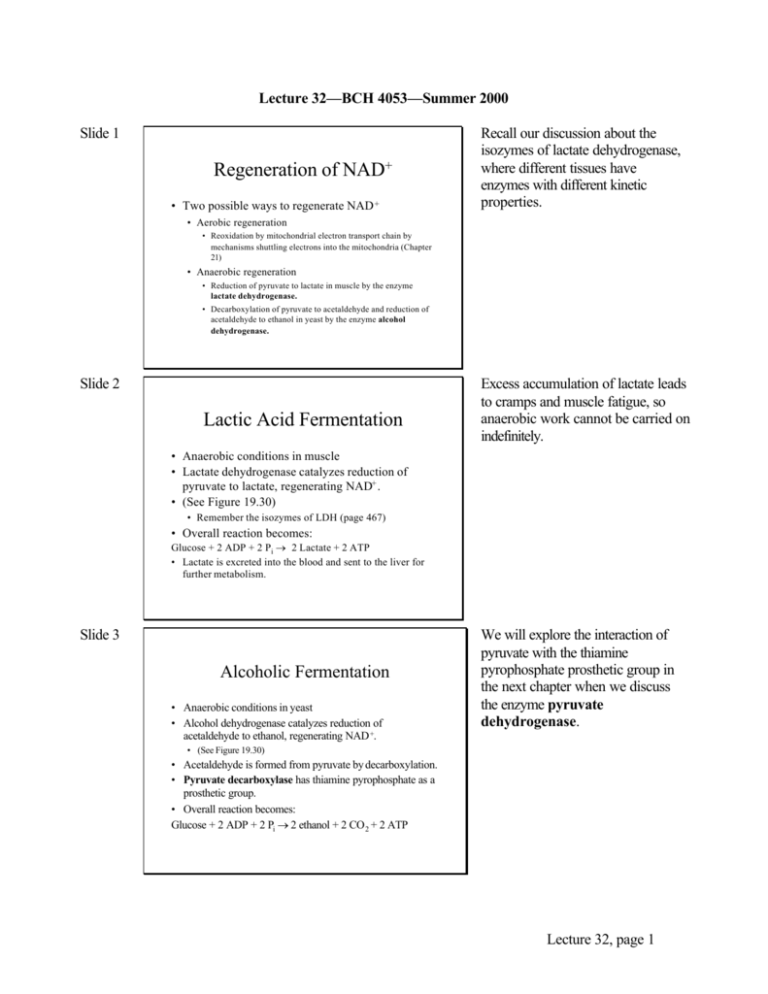
Lecture 32—BCH 4053—Summer 2000 Slide 1 Regeneration of NAD+ • Two possible ways to regenerate NAD + Recall our discussion about the isozymes of lactate dehydrogenase, where different tissues have enzymes with different kinetic properties. • Aerobic regeneration • Reoxidation by mitochondrial electron transport chain by mechanisms shuttling electrons into the mitochondria (Chapter 21) • Anaerobic regeneration • Reduction of pyruvate to lactate in muscle by the enzyme lactate dehydrogenase. • Decarboxylation of pyruvate to acetaldehyde and reduction of acetaldehyde to ethanol in yeast by the enzyme alcohol dehydrogenase. Slide 2 Lactic Acid Fermentation Excess accumulation of lactate leads to cramps and muscle fatigue, so anaerobic work cannot be carried on indefinitely. • Anaerobic conditions in muscle • Lactate dehydrogenase catalyzes reduction of pyruvate to lactate, regenerating NAD+ . • (See Figure 19.30) • Remember the isozymes of LDH (page 467) • Overall reaction becomes: Glucose + 2 ADP + 2 Pi → 2 Lactate + 2 ATP • Lactate is excreted into the blood and sent to the liver for further metabolism. Slide 3 Alcoholic Fermentation • Anaerobic conditions in yeast • Alcohol dehydrogenase catalyzes reduction of acetaldehyde to ethanol, regenerating NAD +. We will explore the interaction of pyruvate with the thiamine pyrophosphate prosthetic group in the next chapter when we discuss the enzyme pyruvate dehydrogenase. • (See Figure 19.30) • Acetaldehyde is formed from pyruvate by decarboxylation. • Pyruvate decarboxylase has thiamine pyrophosphate as a prosthetic group. • Overall reaction becomes: Glucose + 2 ADP + 2 Pi → 2 ethanol + 2 CO 2 + 2 ATP Lecture 32, page 1 Slide 4 Overall Energetics of Glycolysis • Three steps are far from equilibrium. • Hexokinase • Phosphofructokinase • Pyruvate kinase • (See Figure 19.31) Slide 5 Fate of Glucose Carbon Atoms • To interpret isotopic tracer experiments, it is important to understand what happens to each carbon atom of glucose. • Practice labeling a carbon of glucose and tracing the label through the pathway. Slide 6 Fate of Glucose Carbon Atoms, con’t. 1 H 1 CH2OP CHO 2 2C O 3 CH2OH OH 3 HO H O 4 H OH 5 H OH 6 CH OH 2 Yields and or H OH 6 4 5 H O HO HO 1 H 3 H 4 CH 5 HC OH 6 CH2 OP 2 OH H O 3,4 C OH 2,5 C O 1,6 CH3 OH Lecture 32, page 2 Slide 7 Other Sugars in Glycolysis • Mannose is phosphorylated and isomerized to fructose-6-phosphate. • Fructose is phosphorylated to fructose-1phosphate, which is acted on by a special aldolase. (See Figure 19.32) • The regulatory enzyme PFK is bypassed. • Galactose is slightly more complicated. Slide 8 Metabolism of Galactose • Phosphorylation at C-1 • Transfer of UDP from UDP-Glc to form Glc-1-P and UDP-Gal • Epimerization of UPD-Gal to UDP-Glc • See Figure 19.33 • Galactosemia is from a defect in the transferase. • Rarer forms of the disease involve defects in galactokinase or the epimerase. In galactosemia, galactose cannot be metabolized, and its accumulation causes cataracts, neurological disorders, and liver problems. Prevention of the disease consists of removing galactose and lactose from the diet. In adults, another enzyme for activating galactose-1phosphate with UTP alleviates the problem. Slide 9 Metabolism of Glycerol • Glycerol is formed by hydrolysis of triglycerides. • Glycerol kinase forms glycerol-3-phosphate • Glycerol phosphate dehydrogenase converts it to dihydroxyacetone phosphate, a glycolytic intermediate. • (See Figure 19.36) Lecture 32, page 3 Slide 10 Chapter 20 The Tricarboxylic Acid Cycle Slide 11 TCA Cycle aka Krebs Cycle and Citric Acid Cycle • Occurs inside mitochondria (the matrix) • Overall reaction: Acetyl-CoA + 3 NAD + + CoQ + GDP + Pi ↓ 2 CO2 + 3 NADH + CoQH2 + GTP + CoASH Mitochondria have two membranes: inner and outer. The outer has pores and is permeable to many things. The inner is a permeability barrier which requires specific transporters for most compounds to move across it. The matrix is inside the inner membrane. • Bridging reaction required that converts pyruvate to acetyl-CoA. Slide 12 Bridging Reaction: Pyruvate → Acetyl-CoA The reaction is an oxidative decarboxylation of an alpha keto acid. • Catalyzed by Pyruvate Dehydrogenase • Overall reaction: Pyruvate + CoASH + NAD+ → Acetyl -CoA + NADH + CO2 • Two cosubstrate coenzymes: • Coenzyme A and NAD+ • Three prosthetic group coenzymes: • thiamine pyrophosphate, lipoic acid, FAD Lecture 32, page 4 Slide 13 Note the first subunit has the same name as the overall enzyme. Pyruvate Dehydrogenase • Multienzyme complex (See Figure p.646) • Multiple copies of three enzymes, each with a prosthetic group: Pyruvate Dehydrogenase Dihydrolipoyl PDH Thiamine Pyrophosphate TA Lipoic Acid Transacetylase Dihydrolipoyl Dehydrogenase 24 dimers 24 subunits (cubic core) DLD FAD 12 subunits Slide 14 Pyruvate Dehydrogenase, con’t. • Mechanism involves two covalent intermediates with the enzyme: • Addition of pyruvate to TPP and loss of CO2 forms hydroxyethyl TPP. • (This same intermediate is formed by pyruvate decarboxylase in yeast alcoholic fermentation). • Transfer of hydroxyethyl group to lipoic acid to form acetyl lipoic acid. • (This is an internal oxidation -reduction: acetaldehyde is oxidized, lipoic acid is reduced.) • See mechanisms page 646. Slide 15 Pyruvate Dehydrogenase, con’t. • Note the role of lipoic acid as a swinging arm that can move to bind to three different active sites. It cycles through three chemical forms. • The overall reaction is irreversible. • The enzyme is found inside the mitochondria. Therefore pyruvate must cross the mitochondrial membrane. Lecture 32, page 5