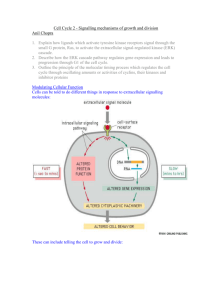
Cell Proliferation and Its Regulation
advertisement

Katherine Hyland, PhD Cell Proliferation and Its Regulation (Biochemistry/Molecular Biology Lecture) OBJECTIVES • Describe the key properties of stem cells. • List the four phases of the cell cycle and describe what happens in each phase. • Name the four cyclin-Cdk complexes that drive the human cell cycle and explain how the timing of their function is regulated. • Diagram the pathway by which the G1 Cdk activates the G1/S Cdk. Describe the molecular events that take place at each step of the pathway, and explain why they are important for the proliferation of normal and cancer cells. • Name the two classes of Cdk inhibitors and the cyclin-Cdk complexes they inhibit. • Describe the general nature of cell signaling networks that allow cells to interpret information from numerous extracellular signals. • Describe three classes of receptor proteins in the plasma membrane, and explain how they transmit extracellular signals to the cell interior. • Diagram the pathway leading from the binding of epidermal growth factor (EGF) to the EGF receptor to activation of the cyclin D gene. Describe the molecular events that take place at each step of the pathway. • Describe the Wnt signaling pathway and its effect on cell proliferation. • Describe in molecular terms how TGFß inhibits cell division. • Describe how apoptosis can be triggered by either extracellular or intracellular signals. • Explain how the balance between pro-apoptotic and anti-apoptotic proteins determines whether a cell will die. • Explain how p53 causes cell cycle arrest and apoptosis. • Describe the spindle assembly checkpoint and the molecular function of the Mad2 protein. • Describe how selective proteolysis is achieved by the cell. KEY WORDS anaphase anaphase-promoting complex/cyclosome (APC/C) antimitogen APC (adenomatous polyposis coli) apoptosis ß-catenin Bcl-2 caspase Cdk inhibitor cell cycle checkpoint chromosome segregation cyclin 35 Mad2 protein MEK metaphase mitogen mitogen activated protein kinase (MAPK) mitotic spindle Myc p16 p21 p27 p53 progenitor cell proteasome Cell Proliferation and its Regulation KEY WORDS (Continued) cyclin-dependent kinase (Cdk) cytochrome c cytokinesis Raf Ras receptor tyrosine kinase E2F transcription factor epidermal growth factor (EGF) Grb2 growth factor GTPase-activating protein (GAP) GTP-binding protein (G-protein) guanine nucleotide exchange factor (GEF) Her2/neu kinetochore SH2 domain sister chromatid sister chromatid cohesion Sos spindle pole stem cell survival factor terminal differentiation transforming growth factor ß (TGFß) Rb protein Wnt protein Optional reading: Alberts et al. Molecular Biology of the Cell; 5th Edition, Garland Science, 2008. Chapter 17: Cell Cycle; Chapter 18: Apoptosis. Kumar, Abbas, Fausto and Mitchell. Robbins Basic Pathology; 8th Edition, Elsevier/Saunders, 2007. Chapter 6 - Neoplasia: Cell Cycle, pp 188-198. I. INTRODUCTION Cell proliferation produces two cells from one, and it requires cell growth followed by cell division. Uncontrolled cell proliferation is a hallmark of cancer. As described in the overview lecture of cancer biology, multiple mutations that accumulate in somatic cells over many years eventually remove an elaborate set of controls that would otherwise prevent cancer cells from dividing unchecked. In this lecture, we will focus on the normal mechanisms that allow nearly all of the billions of cells in our body to proliferate only when they should. These mechanisms are subverted in cancer cells, and it is impossible to understand cancer without first understanding the controls that keep the vast majority of the 1014 cells (100,000 billion cells) that form the human body from misbehaving. In normal tissues, cell proliferation is generally restricted to cells that replenish the tissue. Most tissues are thought to contain stem cells that have this replenishment function (Figure 1). Stem cells are self-renewing cells that can divide asymmetrically to yield a new stem cell and a progenitor cell. Progenitor cells may or may not undergo further divisions, ultimately leading to terminal differentiation. Once cells have terminally differentiated, they have a specialized function and are no longer dividing. Most tissues are made up of such non-dividing cells. Thus proliferation is normally tightly controlled so that only particular cells in the body are dividing. Cell number is dependent not only on cell proliferation, but also on cell death. Programmed cell death, or apoptosis, is the process by which excess or damaged cells in the body are removed. Apoptosis is an extensive, ongoing process in our bodies. It is the balance between the production of new cells and cell death that maintains the appropriate 36 Katherine Hyland, PhD Stem Cell (self-renewing) Progenitor (Dividing) Figure 1. Stem Cells. Stem cells are self-renewing cells. They can divide asymmetrically to produce a new stem cell (indicated by a circle) and a progenitor cell. Progenitor cells divide to produce cells that undergo terminal differentiation to produce the mature cells that make up a tissue or organ. Terminally Differentiated Cells (Non-Dividing) number of cells in a tissue (referred to as homeostasis). Apoptosis is also a key mechanism by which cancer-prone cells are eliminated. Both normal apoptotic processes and normal cell mechanisms that control proliferation usually need to be altered to produce enough abnormal cell proliferation to cause cancer. II. THE CELL CYCLE Cell division occurs in defined stages, which together comprise the cell cycle. In terms of the genetic material, cells must replicate their chromosomal DNA once every cell cycle and segregate the sister chromatids produced by DNA replication to yield two genetically identical daughter cells (Figure 2). During DNA replication, cohesion proteins attach the replicated sister chromatids to each other, holding them together. This sister chromatid cohesion is critical for the subsequent alignment of each pair of sister chromatids on the mitotic spindle (see below), and it is therefore essential for the subsequent segregation of one (and only one) chromatid of each pair into each of the two daughter cells. 14 The cell division cycle is broken up into four stages: G1, S, G2 and M (Figure3). DNA replication occurs during S (“synthesis”) phase. DNA packaging, chromosome segregation and cell division (cytokinesis) occur in M (mitosis). S phase and M phase are separated by Gap phases. G1 is the gap between M and S. Cell growth is one of the Thompson & Thompson GENETICS IN MEDICINE important events of G1. The transition from G1 to S is the critical control point in the cell G1 (10-12 hr) M G2 (2-4 hr) S (6-8 hr) Telomere Centromere Telomere Sister chromatids Figure 2-9 ■ A typical mitotic cell cycle, described in the text. The telomeres, the centromere, and sister chromatids are indicated. 37 spend a long time, days or years, in G1. In fact, some cell types, such as neurons and red blood cells, do not divide at all once they are fully differentiated; rather, The two sister chromatids are held together physically at the centromere, a region of DNA that associates with a number of specific proteins to form the kinetochore. Figure 2. The chromosome cycle. A keyeach chromoThis complex structure serves to attach purpose of cell division is the duplication some to the microtubules of the mitotic spindle and to ofgovern the genetic materialmovement carried onduring mitosis. DNA chromosome chromosomes and its accurate segregation throughout synthesis during S phase is not synchronous such that each daughter cell acquires onechromosome; all chromosomes or even within a single rather, alongchromatid. each chromosome, it begins at hundreds copy of each (Reproduced to thousands of & sites, called origins of DNA from Thompson Thompson Genetics in replication. Individual7th chromosome segments have Medicine, edition, Nusbbaum, et al,their own characteristic replicationfrom during the 6- to 8-hour p.14, 2007,time withofpermission Elsevier.) S phase. By the end of S phase, the DNA content of the cell has doubled, and each cell now contains two copies of the diploid genome. After S phase, the cell enters a brief stage called G2 . Throughout the whole cell cycle, ribonucleic acids and proteins are produced and the cell gradually enlarges, eventually doubling its total mass before the next mitosis. G2 is ended by mitosis, which begins when individual chromosomes begin to condense and become visible under the microscope as thin, extended threads, a process that is considered in greater Cell Proliferation and its Regulation Figure 3. The cell cycle. Reproduced with permission from Alberts et al. Molecular Biology of the Cell. Garland Publishing, 2002. cycle. G2 is the gap between S and M, and provides time for proofreading to ensure DNA is properly replicated and packaged prior to cell division. G0 or quiescence occurs when cells exit the cell cycle due to the absence of growth-promoting signals or presence of prodifferentiation signals. Ordered progression through each phase is intricately regulated through both positive and negative regulatory signaling molecules. The G1, S, and G2 phases comprise interphase, which accounts for most of the time in each cell cycle. The M phase, mitosis, is relatively short (approximately 1 hour of a 24 hour cell cycle). Mitosis is itself divided into several steps, described below. (For a review of mitosis, see the Mitosis and Meiosis online module on iROCKET.) 1. Assembly of the mitotic spindle: At the very beginning of M phase (called prophase), the chromosomes condense while the cytoplasmic microtubules are being reorganized to build a bipolar mitotic spindle. Its purpose is to accurately segregate the two sister chromatids to opposite poles of the cell. 2. Steps leading to metaphase: The nuclear envelope then breaks down, allowing the sister chromatids, which are attached to each other through sister chromatid cohesion, to become linked to the microtubules via attachment sites on each chromatid called kinetochores. Kinetochores are protein-DNA complexes in which proteins that can capture microtubules are held tightly by DNA sequences at the centromere on each sister chromatid pair. The other end of a spindle microtubule is attached to a centrosome (the major microtubule organizing center in the cell, also called the spindle pole body), which has duplicated by this time to form the two spindle poles. Because the two kinetochores on each pair of sister chromatids are attached to opposite spindle poles, they are under tension due to pulling forces that are attempting to move them to opposite poles. Eventually, the balance between these forces causes each chromosome to line up near the center of the spindle, which marks the metaphase stage of mitosis (Figure 4). 3. Anaphase: After all the chromosomes achieve bipolar attachment to spindle microtubules in metaphase, sister chromatid cohesion is rapidly dissolved. As a result, the pulling forces of the microtubules cause the two sister chromatids to move rapidly to the opposite poles (Figure 5). 4. Cytokinesis: After sister chromatids segregate to opposite poles, cells physically divide into two daughter cells through a process that involves pinching in of the plasma membrane (Figure 6). 38 Katherine Hyland, PhD Figure 4. The mitotic spindle at metaphase. All of the chromosomes are lined up at the equator of the spindle. Reproduced with permission from Alberts et al. Molecular Biology of the Cell. Garland Publishing, 2008. Figure 5. Anaphase. Only three pairs of sister chromatids are shown; however, in a diploid cell, this occurs simultaneously for all 46 human chromosomes (that is, for 46 pairs of sister chromatids). (Reproduced with permission from Alberts et al. Molecular Biology of the Cell. Garland Publishing, 2008.) Figure 6. Cytokinesis. After the two sister chromatids are segregated to opposite poles, cells undergo cytokinesis by an organized pinching in of the plasma membrane. (Reproduced with permission from Alberts et al. Molecular Biology of the Cell. Garland Publishing, 2008.) 39 Cell Proliferation and its Regulation III. CELL CYCLE CONTROL: ACTIVATORS and BRAKES How is the cell cycle controlled? The mechanisms of regulation can be broken down into two parts: First, how is the cell cycle regulated so that the different phases occur in the correct order? Second, how do extracellular signals activate or inhibit the cell cycle? This section addresses the first question, the next section (IV) addresses the second. Not until the 1980s was it discovered that a special regulatory system acts like the controller on a washing machine to drive the cell through each of its stages. This regulatory system is more than a billion years old, and most of its central components are essentially the same in single-celled eukaryotes such as yeasts and humans. This has made it possible to use the readily accessible yeast cell to dissect many of the details that underlie the normal regulatory mechanisms that control the growth of the cells in our bodies. A. Cyclin Dependent Kinases: The core activators of the cell cycle control system. The events that occur in each part of the cell cycle are carried out by specific proteins, and these proteins must be synthesized or activated at the correct time in the cycle. For example, before DNA synthesis can begin, the enzymes that produce the nucleotides used in DNA synthesis must be activated. This occurs late in G1 phase. (Remember Nucleotide Metabolism? See lecture from M&N.) Cell cycle progression is positively regulated by a family of protein kinases called cyclin-dependent kinases (Cdks), which function to turn specific proteins on and off at appropriate times in the cell cycle. Like other protein kinases, Cdks turn proteins on or off by phosphorylating them. Each cyclin-dependent kinase has two subunits - a kinase subunit (the Cdk catalytic subunit) and a cyclin subunit (Figure 7). As a monomer, the Cdk has no enzymatic activity; activation requires association with a cyclin protein, which functions as an allosteric activator. Cyclins were first identified as key cell-cycle regulators when it was observed that they undergo a cycle of synthesis and regulated destruction during each cell cycle. There are several different Cdks and a number of cyclins. The kinase subunits are present throughout the cell cycle, while the cyclin subunits are produced and degraded at specific times in the cell cycle, thus providing temporal regulation of the cyclin-Cdk complex. As the cyclin subunit is produced, it binds to the kinase subunit and activates it. The cyclin subunit also targets its kinase partner to specific protein substrates. The key cyclin-Cdk complexes that drive the human cell cycle are listed in Table I. The cell cycle can be viewed as a Cdk cycle (Figure 8). Activation of G1-Cdks by cyclin D turns on the events that occur in the early phase of G1. One of these is synthesis of cyclin E. As cyclin E is made, it binds to Cdk2, forming G1/S-Cdk. As the G1/S-Cdk activity accumulates to a critical threshold, it triggers the transition from late G1 into S phase. Cyclin A is made in S phase. It also binds to Cdk2, forming the S-Cdk that is required for DNA synthesis. Cyclin B is made during S phase and G2. As it is made, it binds to Cdk1 forming M-Cdk. When M-Cdk reaches a threshold activity, it triggers the transition from G2 into the prophase stage of mitosis. 40 Katherine Hyland, PhD Figure 7. Cyclin-dependent kinases (Cdks). Cdks are the key regulators of the cell division cycle in organisms as diverse as baker’s yeast and humans. Cyclin-dependent kinases have two subunits, the kinase (often simply called the Cdk) and a regulatory protein called a cyclin. Table I. The four key cyclin-Cdks that drive the cell cycle Figure 8. The cell cycle as a Cdk cycle. Different phases of the cell cycle are driven by different cyclinCdk complexes. In this simplified view, only a G1/S-Cdk, a S-Cdk and a M-Cdk are shown. These act in sequence, as each cyclin protein is produced, to program the following critical events: the G1-S transition known as Start, S phase (DNA synthesis), and the start of M phase (mitosis). In addition, as described in the text, a G1-Cdk activated by cyclin D phosphorylates the Rb protein to produce cyclin E, which is required for G1/S-Cdk activity. Note that the activity of each Cdk disappears rapidly at a specific time in the cell cycle (as the specific cyclin protein is degraded). The APC/C is a large protein complex that controls a proteolytic process required for the separation of sister chromatids at anaphase. (Reproduced with permission from Alberts et al. Molecular Biology of the Cell. 5th Edition, Garland Publishing, 2008; Fig. 17-16, p. 1062) 41 Cell Proliferation and its Regulation B. G1 regulation: How the G1-Cdk turns on the G1/S Cdk During G1, cells prepare for DNA replication. They must synthesize proteins necessary to replicate their genome, and then assemble the various components of the DNA replication machinery onto the origins of replication. This is coordinated with nutrient and growth factor availability to ensure the cell is in an environment that supports cell division. The G1 phase of the cell cycle is unique in that it represents the only time where cells are sensitive to signals from their extracellular environment. Cells require growth factor-dependent signals up to a point in late G1, referred to as the “restriction point” or Start, after which the transition is made into S phase. The transition between early G1 and late G1 (“Start”) illustrates one way that cyclin-dependent kinases regulate the progression of the cell around the cell cycle. This is a crucial control point that is often dysregulated in cancer. In order to move from early G1 to late G1, the cell must synthesize cyclin E. Transcription of the cyclin E gene requires a transcription factor called E2F. In cells that are not proliferating and in cells that are in early G1, the E2F transcription factor is bound to the promoter for the cyclin E gene, but it is inhibited by a protein that binds it, called Rb. (Rb stands for Retinoblastoma, a childhood tumor of the retina – more on this in the Tumor Suppressor and Oncogene lecture). Rb is a nuclear phospho-protein that plays a key role in regulating the cell cycle. It exists in an active underphosphorylated state and an inactive hyperphosphorylated state. In its active state, Rb serves as a brake that prevents advancement of cells from G1 to S phase. When G1-Cdk activity increases near the middle of G1, G1-Cdk phosphorylates the Rb protein and inactivates it (Figure 9). Inactive phosphorylated Rb releases from E2F and allows transcription of the cyclin E gene to take place. The cyclin E protein binds to the Cdk2 kinase to form the G1/S-Cdk. E2F also transcribes a number of other genes important for S phase, including the genes for DNA polymerase and thymidylate synthase. A. Molecular Events B. Control Relationships G1-Cdk OFF Cdk4-cyclin D (G1-Cdk) Rb E2F Rb cyclin E gene promoter OFF E2F G1-Cdk ON Rb E2F promoter cyclin E P Cdk2 cyclin E gene Cdk2-cyclin E (G1/S-Cdk) ON Figure 9. How the G1-Cdk activates the G1/S-Cdk. The G1-Cdk (Cdk4-cyclin D) phosphorylates the Rb (Retinoblastoma) protein releasing it from transcription factor E2F. E2F can now activate transcription of cyclin E, which in turn results in the production of cyclin E protein and formation of the G1/S Cdk. In describing signaling systems, it is common to use an arrow to indicate an activation and the T-shaped symbol to indicate an inhibition. 42 Katherine Hyland, PhD Importantly, the production a cyclin E, and thus CDK2-cyclin E activity, represents the transition from mitogen-dependent to mitogen–independent cell cycle progression (or passage through “start”), irreversibly committing the cells to enter S phase. Once cells enter S phase, they are committed to divide without additional growth factor stimulation. As we will discuss in later lectures, cells that acquire mutations that obviate the need for mitogen- dependent signals will bypass this crucial control point. C. Brakes on the cell cycle: Cdk inhibitors The Rb protein can be viewed as a “brake” on the cell cycle because it prevents the transcription of the gene for cyclin E by inhibiting E2F. Three other proteins that act as “brakes” on the cell cycle are the Cdk inhibitors p16, p21, and p27. These act by binding directly to Cdk-cyclin complexes and blocking their protein kinase activity (Figure 10). Cdk inhibitors fall into two classes: specific and general. The Ink4 (inhibitors of Cdk4) family of proteins, including p16, bind exclusively to and inhibit the G1 Cdks, Cdk4/6-cylin D. The Cip/Kip family of Cdk inhibitors, including p21 and p27, bind to a broad range of Cdk-cyclin complexes, shutting off the cell cycle at multiple points. Functionally, p21 and p27 appear to mainly inhibit Cdk2 complexes. As will be discussed in the Tumor Suppressor Gene and Oncogene lecture, alterations in these inhibitor proteins play an important role in cancer. Why is the cell cycle controlled by both activators (e.g. cyclins) and inhibitors (e.g. Rb, p16, p21, p27)? As we will see, it helps each cell to respond to multiple inputs, so that it enters the cell cycle only when the correct combination of conditions are present. The control of cycle entry by both growth activating and growth inhibiting signals is part of a “fail-safe” system for insuring that cell proliferation only occurs when it is useful to a multicellular organism like ourselves. Without a complex control system of this type, humans could not exist, because we would all get cancer at a very early age (probably in utero). Figure 10. How Cdk inhibitors bind to and inactivate Cdks. (Reproduced with permission from Alberts et al. Molecular Biology of the Cell. Garland Publishing, 2008. 43 Cell Proliferation and its Regulation IV. CONTROLLING PROLIFERATION: MITOGENS, ANTI-MITOGENS, and CELL SIGNALING A. General Principles of Cell Signaling Cell signaling processes are central to all of human biology and medicine. Although the details of cell signaling pathways can become very complex, the big picture of cell signaling (e.g. transmitting information from the extra-cellular environment into the cell so it can respond appropriately) is straightforward. Signaling pathways are built from a limited set of molecules and molecular mechanisms (e.g. phosphorylation or proteolysis) that allow for communication within and between cells. The underlying molecular mechanisms used in signaling pathways show a number of common properties. In particular, they allow signaling proteins to undergo switch-like activation from an inactive to an active state (for example by receptor clustering, GTP-binding to Ras proteins, and stabilization of β catenin, as described below) and they can also be readily reversed (e.g. by receptor down-regulation, hydrolysis of bound GTP, and β catenin degradation). Dr. Fulton introduced the subject of signaling last year, and it is important to review that material for this block (see lectures on Protein Function and Signaling from Prologue). The introductory lectures focused on one of the two major classes of cell surface receptor proteins present in all cells: the G-protein-linked receptor family. The other major class is referred to as the enzyme-linked receptor family. This class includes receptors linked to protein kinases, which fall into two subgroups: the receptor tyrosine kinases (RTKs) and the receptor serine/threonine kinases. An example of each is discussed below. B. Mitogens and Anti-Mitogens Non-dividing cells exist in phase called G0 (G zero). G0 cells can re-enter the cell cycle in G1 when stimulated by mitogens, which are extracellular proteins that stimulate cell proliferation by directly controlling the entry of cells into the cell cycle. (For historical reasons, mitogens are often loosely referred to as growth factors. Although it is best to reserve the latter term for those signaling molecules that actually induce cell growth, i.e. the increase in cell mass, these terms are often used interchangeably). Conversely, cells can be arrested in G1 via the action of Ligand Binding Domain EGF Kinase Domain P SH2 Tyrosine residue SH3 Sos Grb2 (adaptor protein) 44 Figure 11. Activation of the Epidermal Growth Factor (EGF) receptor tyrosine kinase. EGF binds to the EGF receptor through an extracellular ligand binding domain, leading to dimerization of the receptor. Dimerization causes one subunit to phosphorylate the other (transphosphorylation) on specific tyrosine residues. The SH2 domain of the Grb2 adaptor protein then binds to the region of the EGF receptor containing the phosphorylated tyrosines. Grb2 in turn, uses its second common protein domain, called SH3, to bind to another protein called Sos. Grp2 is known as an adapter protein, since it function to hold two other proteins together. Sos is a member of a large family of proteins that regulate G proteins (GTP-binding proteins) by causing the exchange of a tightly bound GDP molecule for GTP (see Figure 14). Katherine Hyland, PhD anti-mitogens (proteins that inhibit the activity of mitogens). Many mitogens and a smaller number of anti-mitogens are known. We will discuss one example of each: the mitogen epidermal growth factor (EGF) and the anti-mitogen transforming growth factor ß (TGFß). The receptors for these factors are both enzyme-linked receptors. The EGF receptor, or EGFR, is an example of a receptor tyrosine kinase (RTK), and the TGFß receptor is a receptor serine/threonine kinase, What are the normal functions of these factors? One function of EGF is to promote wound healing. After a wound is formed, epidermal and inflammatory cells secrete EGF and other growth factors. It signals cells at the margins of the wound to proliferate so that the wound may be healed. TGFß acts as a brake to this process so that the proliferation is coordinated with other aspects of wound healing. C. The EGF Signaling Pathway The EGF receptor belongs to the ErbB family of RTKs, which has four members capable of homo– or heterodimerization. Each receptor heterodimer can respond to a distinct set of extracellular ligands and has different intracellular signaling properties. Interestingly, another member of the ErbB family, the ErbB2 receptor (also called HER2/neu) lacks intrinsic growth factor-binding activity. Consequently, in normal cells HER2/neu must function as part of a heterodimer with another ErbB family member, such as EGF. (More about HER2/neu and its role in breast cancer in later lectures.) EGF functions by binding to the extracellular domain of EGF receptor, a cell surface protein with a single transmembrane domain (Figure 11). The cytoplasmic domain of the receptor is the protein tyrosine kinase. When EGF binds to its receptor, the receptor forms a dimer in which one subunit phosphorylates the other (transphosphorylation) on particular tyrosine residues in the cytoplasmic part of the receptor. These phosphorylated tyrosines serve as binding sites for other cytoplasmic proteins that contain special domains, called SH2 domains. SH2 domains specifically recognize phosphorylated tyrosines and the adjacent amino acids. One protein that binds to phosphotyrosine residues in the EGF receptor is an adaptor protein called Grb2. Grb2, in turn, recruits a protein called Sos. Thus binding of EGF to the EGF receptor recruits both Grb2 and Sos to the intracellular portion of the receptor. Sos GDP GTP Ras-GDP Ras-GTP inactive active GAP GTPase-activating protein 45 Figure 12. Sos is a guanine nucleotide exchange protein (GEF) that activates the Ras protein. Ras is a monomeric GTP-binding protein that is only active in its GTP-bound form. In its GDP bound form, Ras is inactive. When Sos binds to Grb2 at the EGF receptor, it is brought close to membrane-bound Ras-GDP molecules, causing the Ras to release its GDP and bind a GTP in its place. A second common type of protein is a GTPase-activating protein (GAP), which inactivates Ras by promoting its GTP hydrolysis. The cell contains hundreds of monomeric GTP-binding proteins that serve to regulate many different functions. Each is regulated in a similar way by GEFs and GAPs. Cell Proliferation and its Regulation Figure 13. Ras activates the MAP kinase cascade. Ras-GTP binds directly to Raf, which activates its kinase activity. Raf phosphorylates a kinase called MEK (also called MAP kinase kinase). After it has been phosphorylated by Raf, MEK phosphorylates MAP kinase (mitogen activated protein kinase, MAPK). Active MAPK then phosphorylates its target proteins, including transcription factors, stimulating the entry of the cell into the cell cycle. Ras-Raf MEK MEK-P active inactive MAPK MAPK-P inactive active Target Target-P inactive active Sos is a guanine nucleotide exchange factor (GEF). It acts on a small monomeric GTP binding protein, Ras. The Ras protein is bound to the inner surface of the plasma membrane. Like the G-proteins discussed by Dr. Fulton in the Prologue, Ras can exist in two states: an inactive state in which GDP is bound, and an active state in which GTP is bound. Sos activates Ras by promoting the release of its GDP and binding of GTP (Figure 14). Recruitment of Sos to the plasma membrane where Ras is located results in the activation of Ras. Ras can be returned to its inactive form through the hydrolysis of GTP to GDP. This step occurs when a GTPase-activating protein (GAP) binds Ras and induces the hydrolysis of its GTP (see Figure 12). In its GTP-bound (active) state, Ras turns on a protein kinase cascade, in which protein kinases sequentially activate each other through phosphorylation (Figure 13). Active Ras binds to and activates a protein kinase called Raf. In turn, Raf phosphorylates and activates another kinase called MEK (MAP kinase kinase). MEK in turn phosphorylates and activates mitogen-activated protein kinase, MAP kinase. This chain of phosphorylation events is called the MAP kinase cascade. MAP kinase phosphorylates gene-specific transcription factors in the cell nucleus that bind to the promoters of genes and promote cell proliferation. One important transcription factor that is up-regulated by the MAP kinase cascade is Myc, which is the product of the c-MYC gene. One of the targets of transcription factors that are activated by the MAP kinase cascade is the cyclin D gene. Thus, a multi-tiered pathway connects the presence of a mitogen (EGF) outside the cell to increased expression of a key component of the cell cycle control machinery (the cyclin D gene) in the nucleus (Figure 14). Increased expression of the cyclin D gene leads to the activation of G1-Cdk, pushing the cell to proliferate, as explained previously. MAPK Transcription Factors(e.g. Myc) cyclin D Figure 14. Activation of MAP kinase leads to the transcription of cyclin D. MAPK phosphorylates transcription factors. This in turn leads to the transcription of the Myc gene, which itself encodes a transcription factor for the cyclin D gene. 46 Katherine Hyland, PhD D. Wnt signaling The Wnt proteins are mitogens analogous to EGF. They function in a signaling pathway that regulates cell proliferation by controlling proteolysis of a key signaling protein (Figure 15). The Wnt signaling pathway plays a central role during embryonic development, and also serves important functions in adults. For example, Wnt signaling is necessary for the proliferation of stem cells in the proliferative zones in the gut epithelium (the crypts that lie between the microvilli of the epithelium) (Figure 16). Colon cancer is almost invariably associated with the hyperactivation of this pathway in an early step of tumor evolution. As illustrated in Figure 15, Wnt proteins bind to a cell surface receptor called Frizzled. Frizzled controls the stability of a protein called ß-catenin, which functions together with a protein called TCF to form a transcription factor that activates the promoter of the cyclin D gene. When Wnt is bound, Frizzled turns off a protein kinase called GSK-3. GSK-3 normally functions to promote the degradation of ß-catenin, thus preventing it from activating the cyclin D promoter. Phosphorylation of ß-catenin by the protein kinase GSK-3 results in its degradation. However, GSK-3 can only phosphorylate ß-catenin when ß-catenin is bound to a protein called APC (adenomatous polyposis coli). Thus, APC is necessary to hold ß-catenin in check, and loss or inactivation of APC is associated with development of colorectal cancer (as described in later lectures). (Note: this APC protein is not to be confused with APC/C, the anaphase promoting complex/cyclosome, to be described later in this lecture.) Wnt Frizzled GSK-3 b-catenin APC P P b-catenin APC degradation TCF APC b-catenin TCF cyclin D Figure 15. The Wnt signaling pathway (see text for details). 47 Cell Proliferation and its Regulation microvillus microvillus high Expression of APC low crypt (proliferating stem cells) Figure 16. Expression of the APC gene in the gut epithelium. Shown is a schematic of a microvillus in the gut epithelium showing the zone of proliferation (crypts) and the gradient of expression of the APC gene, whose protein product inhibits Wnt signaling. Once GSK-3 is inhibited by Frizzled, ß-catenin is no longer degraded, allowing it to associate with TCF and activate the cyclin D promoter and promote cell proliferation. Thus, Wnt signaling promotes cell proliferation through the effect of ß-catenin on cyclin D production. While Wnt proteins are the extracellular growth factors that activate this pathway, cells also control the pathway from within the cell by varying the transcription of the APC gene, whose protein product inhibits the Wnt signaling pathway. For example, in the epithelium of the colon, there exists a gradient of APC expression that is highest in the terminally differentiated nondividing cells in the microvilli and lowest in the proliferating stem cells in the crypts (see Figure 16). (More about the role of APC in colon cancer to come in lectures on Colon Cancer and Familial and Hereditary Cancer Syndromes.) E. TGFß-Smad: An anti-mitogenic pathway Like EGF, TGFß is an extracellular protein that binds a cell surface receptor. However, instead of causing cell proliferation, this molecule causes cells to arrest their cell cycle and enter G0. How does this occur? The TGFß receptor is a transmembrane serine/threonine kinase. Upon binding to TGFß, the receptor phosphorylates proteins in the cytoplasm called Smads (Figure 17). Once phosphorylated, Smad proteins then enter the nucleus and function as transcription factors to turn on specific target genes. A key gene turned on by TGFß is the Cdk inhibitor p21 discussed above. The activation of p21 blocks G1-S transition by inhibiting Cdk2-Cyclin E/A, leading to the arrest of the cell cycle. Thus, TGFß arrests cell division by turning on transcription of the gene for a Cdk inhibitor. V. APOPTOSIS As previously explained, the number of cells in a tissue is controlled not only by cell proliferation, but also by programmed cell death, or apoptosis. For a tissue to stay the same size, cell proliferation and cell death must be perfectly balanced. Apoptosis plays important roles both during development and in mature tissues. For example, during development of a limb, tissue present between the digits must be removed. This occurs through localized apoptosis (Figure 18). 48 Katherine Hyland, PhD TGF� receptor TGF� Smad plasma membrane kinase domain Smad-P p21 Smad-P p27 gene promoter Figure 17. How TGFß arrests cell division. TGFß binds to the TGFß receptor. Binding of TGFß activates the receptor’s intracellular protein kinase domain, leading to phosphorylation of Smad proteins on serine and threonines. Phosphorylated Smads enter the nucleus and bind to promoters of genes to control transcription. A key target is the p21 gene. The p21 protein in turn inhibits cyclin E/A- cdk2 complexes, thus leading to cell cycle arrests. Cdk4-cyclin D Cdk2-cyclin E Cdk2-cyclin A nucleus As described in the Prologue block, the process of apoptosis requires the activation of a special class of proteases inside the cell known as caspases. Caspase molecules normally exist as inactive procaspase molecules in the cell. Procaspase activation is carefully controlled, so that the cell only kills itself when this is appropriate for the success of the organism as a whole. A. Cell-surface death receptors activate an extrinsic apoptotic pathway Procaspase activation can be initiated from outside the cell, as happens in the immune system when T cells kill their target cells by producing a signaling protein called Fas ligand. The Fas ligand binds to its receptor, Fas, on target cells. The cytoplasmic domain of a “death receptor” such as Fas is then triggered to bind adaptor proteins that link the receptor to procaspase-8 molecules. The aggregated procaspase-8 molecules are thereby stimulated to cleave each other, initiating a proteolytic cascade that leads to apoptosis (Figure 19A). B. An intrinsic apoptotic pathway depends on mitochondria When cells are stressed (e.g., hypoxia), damaged (e.g., unrepaired DNA damage), or become abnormal in other ways, they can activate apoptosis from inside the cell by triggering a similar process of procaspase aggregation and activation. In response to stress or damage, pro-apoptotic signals induce mitochondria to release cytochrome c into the cytosol, where it binds and activates an adaptor protein called Apaf-1. This causes Apaf-1 to aggregate into a wheel-like complex called an apoptosome. This aggregate then recruits a set of procaspase-9 molecules, which become activated to trigger a caspase cascade causing cell death (Figure 19B). 49 Cell Proliferation and its Regulation Apoptosis ligand development of limb, tissue present Figure 18. Programmed cell death.FasDuring Fas between the digits is removed by apoptosis. Apoptosis Killer T-cell Target Cell Figure 19. Induction of apoptosis by either extracellular or intracellular signals. (A) Extracellular activation. Adaptor proteins bind the intracellular region of aggregated Fas proteins, causing the aggregation of procaspase-8 molecules. These then cleave one another to initiate the caspase cascade. (B) Intracellular activation. Mitochondria release cytochrome c, which binds to and causes the aggregation of the adaptor protein Apaf-1. Apaf-1 binds and aggregates procaspase-9 molecules, which are activated to trigger a caspase cascade, leading to apoptotic cell death (From Alberts et al., Molecular Biology of the Cell, 2002) 50 Katherine Hyland, PhD Domains Function Examples BH 1, 2, 3 Pro-apoptotic Bak, Bax BH 1, 2, 3, 4 Anti-apoptotic Bcl-2 BH 3 only Pro-apoptotic Bad, Bid, Puma Table 2. Three subclasses of proteins in the Bcl-2 family that control apoptosis by the intracellular (intrinsic) pathway. The release of cytochrome c from the mitochondria is tightly controlled by members of the Bcl-2 family of proteins, all of which contain at least one BH protein domain. Within this family of proteins, there are three sub-classes (Table 2): two subclasses promote apoptosis (the “pro-apoptotic” BH123 proteins, which contain 3 different BH protein domains, and the BH3-only proteins), and one subclass antagonizes apoptosis (the “anti-apoptotic” Bcl-2 proteins). The BH3 domain is the only domain shared by all three subclasses of proteins, and it can mediate a direct binding interaction between one pro-apoptotic protein and one anti-apoptotic protein to form heterodimers. The central players are the BH123 family members, Bak and Bax, which can form channels in the mitochondrial outer membrane that cause cytochrome c and other proteins in the mitochondrion’s intermembrane space to be released into the cytoplasm, thereby activating procaspase-9 via Apaf-1. The anti-apoptotic Bcl-2 proteins appear to bind directly to Bak and Bax to inhibit them, thereby serving to keep the cell alive. The remaining BH3-only pro-apoptotic subclass is composed of a large number of proteins that bind to various subsets of the anti-apoptotic Bcl-2 proteins, forming heterodimers with them. If large enough amounts of the BH3 proteins are present in the right combinations, they will dissociate all of these inhibitors from Bak and Bax, thereby permitting the channel formation and inducing cell death (Figure 20). In summary, it is the balance between the activities of the set of anti-apoptotic Bcl-2 proteins and the two subclasses of pro-apoptotic proteins that determines whether a mammalian cell lives or dies by the intrinsic pathway of apoptosis. This balance is determined through a complex and poorly understood signaling network that continually monitors the state of the cell. For example, only if a cell is in its expected location in the organism will it receive the specific survival signals that it requires to prevent apoptosis. Thus it is not surprising that cancer cells often acquire mutations that allow them to alter the balance between pro- and anti-apoptotic proteins, making it less likely for them to commit suicide even under conditions when normal cells would. VI. p53, THE CELL CYCLE, and APOPTOSIS The cell cycle is controlled at certain stages by checkpoints. These are biochemical mechanisms that stop the cell cycle if certain conditions are not met. One checkpoint is the G1 DNA damage checkpoint. If cells contain unrepaired damage to their DNA, the cell cycle is arrested in G1. This arrest requires a key transcription factor, p53, which is activated by DNA damage (Figure 21). There are three components to the system: 1) a DNA damage sensor, 2) the Mdm2 protein that normally causes p53 to be degraded, and 3) the p53 protein itself. DNA damage causes phosphorylation of p53 and blocks the binding of Mdm2. This leads to the stabilization and accumulation of p53. p53 can then bind to the promoter of the p21 Cdk inhibitor described earlier and activate its transcription, causing p21 to accumulate. The resulting inhibition of Cdks leads to cell cycle arrest. 51 Cell Proliferation and its Regulation Figure 20. How pro-apoptotic BH3only and anti-apoptotic Bcl2 proteins regulate the intrinsic pathway of apoptosis. (A) In the absence of an apoptotic stimulus, anti-apoptotic Bcl2 proteins bind to and inhibit the BH123 proteins on the mitochondrial outer membrane (and in the cytosol not shown). (B) In the presence of an apoptotic stimulus, BH3-only proteins are activated and bind to the anti-apoptotic Bcl2 proteins so that they can no longer inhibit the BH123 proteins, which no become activated an aggregate in the outer mitochondrial membrane and promote the release of intermembrane mitochondrial proteins into the cytosol. Some activated BH3-only proteins may stimulate mitochondrial protein release more directly by binding to and activating the BH123 proteins. Although not shown, the anti-apoptotic Bcl2 protins are bound to the mitochondrial surface. (Reproduced with permission from Alberts et al. Molecular Biology of the Cell. Garland Publishing, 2008. Figure e18-11, p. 1124.) If p53 activation continues for a prolonged period of time, apoptosis ensues. This process kills cells with damaged DNA that remain unrepaired, and serves to remove cells from tissues that may otherwise accumulate mutations that would be passed on to their daughter cells. High levels of p53 are thought to activate apoptosis by increasing the transcription of several genes. One target gene is the BH123 protein Bax, whose gene is directly activated by p53 (Figure 22). In light of the important role p53 plays in preventing unrepaired DNA damage to be passed on to daughter cells, it is not surprising that p53 is found to play a central role in cancer development. In fact, the p53 pathway is mutated in nearly all cancers, thereby allowing damaged DNA to remain in cells as they proliferate (more in the lecture on Tumor Suppressor Genes and Oncogenes). 52 Katherine Hyland, PhD Figure 21. How DNA damage activates p53 and causes cellcycle arrest. DNA damage activates a protein kinase that phosphorylates p53, preventing its degradation. This leads to the production of high levels of the Cdk inhibitor p21. (reproduced with permission from Alberts et al. Molecular Biology of the cell. 5th Edition, Garland Publishing, 2008: Fig 17-63.) Prolonged p53 Activation p53 Figure 22. DNA damage can lead to apoptosis. Prolonged activation of p53 in response to DNA damage results in apoptosis. p53 activates the transcription of several genes involved in apoptosis including that for the pro-apoptotic BH123 protein Bax shown here. BAX gene promoter cytochrome C apoptosis Bax channel 53 Cell Proliferation and its Regulation VII. THE SPINDLE ASSEMBLY CHECKPOINT: THE IMPORTANCE OF REGULATED PROTEOLYSIS IN THE CELL In addition to monitoring the state of DNA in G1 before entering S phase, cells also monitor the state of the cell at several other checkpoints. One, called spindle assembly checkpoint, ensures that mitosis does not proceed beyond metaphase until the spindle is properly assembled. This checkpoint monitors the attachment of spindle microtubules to each kinetochore through the action of the Mad2 protein (Figure 23). There are two key features of the checkpoint: 1) Mad2 associates with kinetochores only when they are not attached to microtubules, and 2) Mad2 becomes activated for arresting mitosis only when bound to such kinetochores. If even one of the 46 human chromosomes is not attached correctly to microtubules, enough Mad2 is activated to keep the cell in metaphase. Only when the spindle has been properly assembled with all of the kinetochores bound to microtubules does Mad2 becomes inactive and allow anaphase to proceed. If there is a problem with spindle assembly, Mad2 will arrest the cell cycle until the problem is resolved. Active Mad2 exerts its effects by blocking the key regulator of the metaphase-toanaphase transition, the anaphase-promoting complex/cyclosome (APC/C). The APC/C is a member of a large family of important enzymes, called ubiquitin ligases, that trigger the regulated destruction of target proteins in the cell. The actual proteolysis is carried out by proteasomes, large protein complexes that pump selective proteins into their interior in order to cleave them into small fragments. As a ubiquitin ligase, the APC/C marks proteins for uptake into proteasomes by covalently adding multiple copies of the small protein called ubiquitin to them. The polyubiquitin chain added to a protein is recognized by the proteasome, causing the protein to be destroyed. One of the destroyed proteins is an inhibitor of the protein that cell cycle arrest microtubules centrosome Mad2 kinetochore Mad2 inactive Sister Chromatids Figure 23. Spindle assembly checkpoint. This checkpoint functions through the action of the Mad2 protein, which binds to kinetochores that have not attached to microtubules. When bound to kinetochores, Mad2 triggers cell cycle arrest. Once microtubules are attached to all of the kinetichores, Mad2 is no longer active and the cell cycle proceeds. 54 Katherine Hyland, PhD cuts the linkages holding the sister chromatid pairs together. The removal of the inhibitor allows the separation of sisters and unleashes anaphase. The S- and M-cyclins are the second major targets of the APC/C. The destruction of these cyclins inactivates the corresponding Cdks (see Figure 8). As a result, the many proteins phosphorylated by Cdks from S phase to early mitosis are dephosphorylated by various protein phosphatases that are present in the anaphase cell. This dephosphorylation of Cdk targets is required for the completion of M phase, including the final steps in mitosis and the process of cytokinesis. Not surprisingly, cells defective in the spindle assembly checkpoint show high rates of aneuploidy because of errors in chromosome segregation during mitosis. Defects in the spindle-assembly checkpoint, and specifically in Mad2, have been associated with tumorigenesis. VIII. THE EVOLUTION OF CELL SIGNALING AND CANCER Now that we have explored the key aspects of normal cell proliferation, we can begin to consider what goes awry in cancer cells. The following section considers theoretical aspects of the evolution of cell signaling and cancer to provide you with context for thinking about cancer development and treatment. (From Dr. Bruce Alberts) A. Elaborate cell signaling mechanisms had to evolve in multicellular organisms to prevent cancer. Various types of evidence suggest that single-celled life was present on the earth 3.5 billion years ago, about a billion years after the earth formed (prokaryotic cells such as bacteria). However, it appears to have required another two billion years to evolve the first multicellular organisms. Initially these were very small aggregates of eukaryotic cells that had learned how to cooperate, with each cell restraining its own growth for the good of the entire aggregate. Although this had the advantage of allowing each type of cell to specialize, it meant that each cell had to send and receive an elaborate set of signals to determine its appropriate behavior, and that fail-safe controls had to evolve to prevent the type of selfish cell behavior that we call cancer. As larger and larger organisms evolved, major improvements to these fail-safe controls had to develop in the form of multiple, largely redundant systems that prevent aberrant cell proliferation. Why? Even with the overlapping set of proofreading mechanisms that allow us to replicate the three billion (3 x 109) nucleotide pairs in the human genome with an error rate of only about one in a billion (10-9), the fact that humans are formed from about 1014 cells means that billions of cells experience mutations every day, potentially disrupting the normal controls on cell growth. Viewed from this perspective, the surprising thing about large multicellular organisms is how infrequently cells misbehave to create a tumor. As we shall see, the reason we do not all die of cancer is that, in general, many different mutations need to accumulate in a single line of cells to cause this disease – perhaps 10 to 20. Obtaining a better understanding the multiple layers of control that are circumvented during tumorigenesis will be key to controlling cancer. Unfortunately, there is still much to learn in this critical area of research. B. Cells integrate the many signals that they receive in deciding whether to survive, grow and divide (proliferate), differentiate, or die (apoptosis). Every cell contains many different cell surface receptor molecules, each of which recognizes a particular molecule at the cell exterior. Some of these bind to signaling molecules that have been secreted by neighboring cells, others bind to protein molecules held in the plasma membrane of tightly opposed adjacent cells, while others bind to the extracellular matrix. All of these signal molecules work in combinations to regulate the behavior of the cell, 55 Cell Proliferation and its Regulation with each of the hundreds or thousands of different cell types in our bodies responding to this babble of signals differently. As shown in Figure 24, an individual cell generally requires multiple signals just to survive. It requires additional signals to grow and divide, and a different set of additional signals to differentiate. If deprived of its required survival signals, a cell will undergo cell suicide (apoptosis). The actual situation is even more complex than illustrated in Figure 24, since some extracellular signal molecules act to inhibit these and other cell behaviors, or even to induce apoptosis. How exactly a cell makes each of the all-or-none decisions illustrated in Figure 24 is not understood in detail. Speaking metaphorically, the decision is analogous to “cell thinking”. Cells integrate the many signals they receive through a “cross-talk” between different intracellular events triggered by different cell surface receptors. Some of the cross talk depends on simple “coincidence detectors”, as in the example shown in Figure 25. Here two different signaling events are needed to activate a single protein inside the cell, because the protein needs to be phosphorylated at two different sites to become active. Thus, the activation of this protein occurs if, and only if, two specific extracellular signals are present simultaneously. But much of the cross talk is more complex and not yet decipherable. Figure 24. How an animal cell depends on multiple extracellular signal molecules. Reproduced with permission from Alberts et al. Molecular Biology of the Cell. Garland Publishing, 2008. 56 Katherine Hyland, PhD Figure 25. Signal integration inside the cell. Here signals A and B each trigger a different intracellular signaling pathway. Both pathways involve the activation of a protein kinase that phosphorylates protein Y, but at a different site. Because both sites must be phosphorylated for protein Y to become activated, protein Y serves as a coincidence detector that indicates that both extracellular molecules A and B are present. (Reproduced with permission from Alberts et al. Molecular Biology of the Cell. Garland Publishing, 2008.) Why is it so important to understand how cells “think”? Cancer can be viewed as a disease in which a cell has accumulated so many changes in its intracellular processes that it has escaped from all of its normal requirements, thinking that it should proliferate and survive independent of its environment. The ideal cancer therapy would be based on an understanding of the exact, highly abnormal intracellular state of the cells in a particular tumor. One might then be able to induce apoptosis in the cancer cells by exposing them to a mixture of two or three specific signaling molecules (or inhibitors of such molecules), with no deleterious effect on normal cells. It is important to keep in mind, however, that each tumor has its own unique set of mutations and aberrant signaling pathways, resulting from a long evolutionary process of random mutation and natural selection during tumor progression. Thus, we should view cancer as a collection of different but related diseases, each of which may require its own specific combination of therapies to treat it. Acknowledgements: Significant contributions to this lecture were made by Hiten Madhani, PhD, Department of Biochemistry and Biophysics, who originally developed this lecture (lecturer 2002 – 2006); and Bruce Alberts, PhD, Department of Biochemistry and Biophysics (lecturer 2007). 57 Cell Proliferation and its Regulation 58