Geometry Molecular compounds have covalent bonds between
advertisement

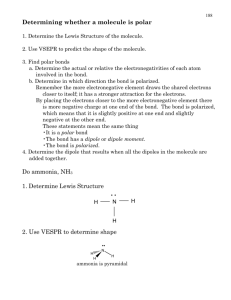
Geometry Molecular compounds have covalent bonds between atoms. Some covalent bonds are polar due to the unequal electron distribution between the two atoms involved in the bond. The bond polarity and molecular shape are both used to determine the polarity of the molecule and the molecule’s aqueous solubility. The validity of these predictions can be experimentally tested by quantitatively measuring the compound’s solubility in water. Objectives 1. Write Lewis Structures 2. Construct molecular models of covalent compounds 3. Determine the molecular geometry from visual inspection of the molecular models 4. Draw the molecular geometry 5. Determine the bond polarity and molecular polarity 6. Predict whether a compound will be soluble in water Prior to lab, read sections of the textbook that discuss molecular geometry bond polarity, and molecular polarity. Chemicals Dilute NH3 CCl4 Equipment Molecular model kits Periodic table Table of Electronegativity values (see textbook) Small test tubes Small stoppers SAMPLE ANALYSIS OF DATA Formaldehyde, CH2O, was studied using the procedures described in this experiment. From the model, the molecular shape for this compound was visually determined to be trigonal planar; bond electronegativity information for this compound is given below. Electronegativity EN Values: Oxygen 3.5, Carbon 2.5, Hydrogen 2.1 Bond Electronegativity Differences between atoms: For C = O bond: 3.5 - 2.5 = 1.0 For C – H bond: 2.5 - 2.1 = 0.4 Data table for CH2O Compound CH2O Molecular geometry a)Trigonal Planar Net dipole points towards oxygen Polar Bonds Polar Molecule Soluble in C6H14 b)Yes c) Yes d) No a. The Lewis structure for this molecule shows two single C-H bonds and a double C=O bond. The central atom, carbon, has 3 bonding regions and 0 nonbonding regions around it. Bonding regions (electron domains) are represented by the number of atoms bonded to the central atom. There are 3 atoms bonded to carbon: 2 Hydrogen atoms and 1 Oxygen atom. Nonbonding regions are represented by lone pairs of electrons located on the central atom. There are 0 lone pairs of electrons about carbon in the Lewis structure. This combination of bonding and nonbonding electron regions results in a trigonal planar shape for the molecule. NOTE: The shape can also be determined visually with molecular models. b. Any bond with an electronegativity difference (greater than zero but less than 2.0) is considered to be a polar covalent bond. ( zero < EN difference < 2.0) c. In order for a molecule to be overall polar, the dipole arrows associated with polar bonds must “add” to make one large dipole within the molecule. To “add/subtract” dipole arrows, one must consider the molecular shape, the symmetry of the molecule, and the strength and direction of the dipoles. In formaldehyde the dipoles for the C - H bonds are much smaller (weaker) than the C = O dipole (based on the difference in EN values). The dipole arrows in the C H bonds are directed at carbon, the more electronegative atom. The dipole arrow for the C=O bond is directed at oxygen, the more electronegative atom. The polarity of the C = O bond cannot be “subtracted” out or "cancelled" by the polarity of the C - H bonds, because the dipoles do not cancel. As a result, an overall dipole exists along the C=O bond and the molecule formaldehyde is therefore a polar molecule. d. The solubility rule is “like dissolves like.” Hexane CH3CH2CH2CH2CH2CH3 (or C6H14) is considered to be a nonpolar solvent. Above in (c), formaldehyde was classified as a polar molecule, therefore formaldehyde is not soluble in nonpolar solvents such as hexane. Formaldehyde is generally soluble in polar solvents, such as water based on the solubility rule "like dissolves like". Directions 1. Write the Lewis structures for each compound. Show resonance. 2. Draw and name the molecular geometry. 3. Using a molecular model kit, make models of each compound under study. Be sure to use a bonding stick for each lone pair about the central atom. Determine the molecular geometry for each compound; record this information in the data table. 4. Using an electronegativity table, determine the presence of polar bonds in each compound; record this information in the table. 5. .Draw each compound with its predicted molecular shape and dipole arrows over the polar bonds (make sure the arrow tip is pointing to the most electronegative atom in the bond). Visually “add” the dipole arrows together (if arrows are converging to common point) or “subtract” the dipole arrows (if the arrows point in opposite directions). Identifying symmetry in the molecule will help with this visualization. 6. If the dipole arrows “add” or do not cancel, the molecule is considered polar. If the dipole arrows "cancel", the molecule is considered nonpolar; record this information in the table. 7. Predict each compound’s solubility in water; record this information in the table. (Remember this rule: "like dissolves like"; polar molecules are soluble in polar solvents; nonpolar molecules are not soluble in polar solvents) Follow-up questions: 1. Test your prediction for ammonia. Using a small disposable pipet, add 1ml. of ammonia NH3 to a small test tube. Add 1 mL. of R.O. water. Stopper the test tube. Invert several times. Is ammonia soluble (miscible) in water? Does this result support your prediction of its solubility? 2. Next test the solubility of carbon tetrachloride. Using a small disposable pipet, add 1 ml. of carbon tetrachloride to a small test tube. Add 1mL. of R.O. water. Stopper the test tube. Invert several times. Is carbon tetrachloride soluble (miscible) in water? Does this result support your prediction of its solubility? Dispose of this solution in the waste container provided. 3. When a can of soda pop is opened, the CO2 gas bubbles escape. Explain why this happens based on the information you have learned in this experiment.