Which Method of Assigning Bond Orders in Lewis Structures Best
advertisement

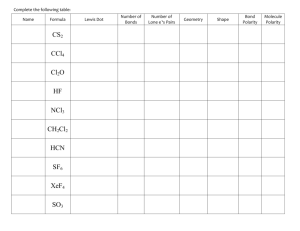
Supplemental Materials 1. Structural parameters for selected nonmetal oxides. Octet rule AO2 A = N or P N2 O4 N2 O3 AO2 A = O or S Formal charge Octet rule SO3 ClO ClO2 Formal charge Supplemental Materials 2a. Structural parameters for selected nitrogen halides and oxohalides. Octet rule ONF3 Formal charge Octet rule O2NX Formal charge Supplemental Materials 2b. Structural parameters for selected halides and oxohalides with Period 3 central atoms. Octet rule Formal charge Octet rule PX5 OAX3 A = N or P SX4 O2SX2 SX6 O3ClX ClX3 O2PCl Formal charge Supplemental Materials 3. Structural parameters for selected nonmetal oxoacids and oxoanions. Octet rule Formal charge Octet rule HNO3 SO42- NO3HClO4 H2SO4 ClO4- HSO4ClO3- Formal charge Supplemental Materials 4. Structural parameters for Group 13 halides and tetrahalide anions. Octet rule Formal charge Brown’s BVM conception of AX3 and AX4- species. AX3 A = B or Al X = F or Cl AX4– A = B or Al X = F or Cl AX 3 AX4 - Supplemental Materials 5 – Systematic drawing of Lewis structures. Lewis structures can be drawn using a systematic, step-by-step approach, as is illustrated below for SO2 (the adjustments required for binary molecules are well known, and so it will not be discussed here). The difference between the octet rule and formal charge systems comes at the end of the usual procedure, when multiple bonds are added. Step 1: Sum the valence electrons. (6 valence e– from S) + 2 x ((6 valence e– from O) = 18 valence electrons Step 2: Determine the central atom. – sulfur (lower electronegativity) Step 3: Draw skeletal structure, connecting central atom to each terminal atom with one bond. Step 4: Place 6 electrons (3 lone pairs) around every terminal, non-hydrogen atom. Step 5: Place any remaining electrons on central atom. Step 6a: Octet rule system only – Convert terminal atom lone pairs to bond pairs until central atom has 8 (or 7, for radicals) valence electrons. Step 6b: Formal charge system only – convert additional terminal atom lone pairs to bond pairs until formal charges are minimized (all zero for neutral molecules). Supplemental Materials 6. Lewis structures of the Group 13 halides (left) and tetrahalide anions (right). Octet rule Formal charge BVM conception Supplemental Materials 7. Bonding in ONF3. .. : F: .. : F: .. .. :O N F .. .. : .. :F .. : .. .. : :O N F .. .. : :F .. : .. :F .. : .. :O .. N :F .. : .. F .. : .. : F: .. :O .. N . : F . . :F .. : ONF3 – 4 resonance structures, N-F bond order ~0.75, N-O bond order ~1.75, total bonding at nitrogen < 4 .. O .. .. : F: P :F .: . .. F .. : OPF3 – 1 structure, P-F bond order ~ 1.0, P-O bond order ~ 2.0, total bonding at phosphorus ~ 5